Abstract
Aims/hypothesis
Displaying immunomodulatory capacities, mesenchymal stem cells (MSCs) are considered as beneficial agents for autoimmune diseases. The aim of this study was to examine the ability of MSCs to prevent autoimmune diabetes in the NOD mouse model.
Methods
Prevention of spontaneous insulitis or of diabetes was evaluated after a single i.v. injection of MSCs in 4-week-old female NOD mice, or following the co-injection of MSCs and diabetogenic T cells in irradiated male NOD recipients, respectively. The frequency of CD4+FOXP3+ cells and Foxp3 mRNA levels in the spleen of male NOD recipients were also quantified. In vivo cell homing was assessed by monitoring 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE)-labelled T cells or MSCs. In vitro, cell proliferation and cytokine production were assessed by adding graded doses of irradiated MSCs to insulin B9–23 peptide-specific T cell lines in the presence of irradiated splenocytes pulsed with the peptide.
Results
MSCs reduced the capacity of diabetogenic T cells to infiltrate pancreatic islets and to transfer diabetes. This protective effect was not associated with the modification of diabetogenic T cell homing, but correlated with a preferential migration of MSCs to pancreatic lymph nodes. While injection of diabetogenic T cells resulted in a decrease in levels of FOXP3+ regulatory T cells, this decrease was inhibited by MSC co-transfer. Moreover, MSCs were able to suppress both allogeneic and insulin-specific proliferative responses in vitro. This suppressive effect was associated with the induction of IL10-secreting FOXP3+ T cells.
Conclusions/interpretation
MSCs prevent autoimmune beta cell destruction and subsequent diabetes by inducing regulatory T cells. MSCs may thus offer a novel cell-based approach for the prevention of autoimmune diabetes and for islet cell transplantation.
Similar content being viewed by others
Introduction
Mesenchymal stem cells (MSCs) are multipotent cells, most of which reside within the bone marrow. These cells, first characterised by Friedenstein and colleagues more than 40 years ago [1], can be induced to undergo differentiation under appropriate conditions into several lineages, including cartilage, bone, adipose and stromal tissues, as reviewed recently [2, 3]. Studies conducted in both humans and animal models have demonstrated that MSCs are capable of long-term engraftment and multilineage differentiation in vivo.
In addition to these characteristics, which support the successful use of allogeneic MSCs in regenerative therapy, MCSs are able to modulate T cell responses and/or induce an immunosuppressive local milieu, which make them attractive potential therapeutic agents for various immunological disorders [4]. The i.v. administration of MSCs prolonged skin graft survival modestly but significantly [5], to an extent similar to that seen in response to the immunosuppressive agents currently in use. MSCs have also recently been demonstrated to suppress several T lymphocyte activities [6] and have been proposed as a possible treatment for acute graft-versus-host disease in humans [7]. Although the mechanisms mediating such effects are only partially understood, it is likely that both cell-to-cell contact and soluble factors are involved [8]. Another property unique to MSCs—a lack of expression of MHC class II and other surface markers such as CD11b, CD14, CD31, CD34 or CD45—results in their failure to initiate CD4+ T cell activation and likely explains how MSCs escape the normal process of alloantigen recognition.
Modulation of autoimmunity is therefore considered a potential novel target for MSC-based treatments. It has recently been reported that MSCs are capable of improving experimental autoimmune encephalomyelitis, the murine counterpart of human multiple sclerosis [9, 10]. In a murine model of rheumatoid arthritis, infusion of MSCs had no beneficial effects on collagen-induced arthritis [11], while another report demonstrated that allogeneic murine MSCs are able to prevent joint tissue damage in rheumatoid arthritis [12]. However, the therapeutic potential of MSCs in type 1 diabetes remains completely unexplored, as reviewed recently by Abdi et al. [13]. The NOD mouse is the foremost model used to assess preventive strategies in type 1 diabetes, as it develops a spontaneous form of autoimmune diabetes that closely resembles the human disease [14, 15]. Beta cell destruction results from progressive islet infiltration by autoreactive T cells and macrophages on a permissive genetic background. In this paper we report on the capacity of MSCs to inhibit autoimmune beta cell destruction in the NOD mouse by induction of regulatory T cells.
Methods
Isolation, culture and characterisation of mouse MSCs
MSCs generated from BALB.B (H2b) mice were a generous gift of E. Simpson (Department of Immunology and Transplantation Biology, Hammersmith Hospital, London, UK) and were cultured as previously described [8]. Before experimental use, the ability of the MSCs to differentiate into adipocytes was tested by adding 10−8 mol/l dexamethasone to the culture medium for 1 week. Oil Red O dye was used to identify adipocytes. The phenotypic properties of MSCs were also determined on the basis of CD105, CD73, CD29 and CD44 expression and the absence of any hematopoietic (i.e. CD45, CD14, CD11c) or endothelial (i.e. CD31) markers. All antibodies for these experiments were purchased from BD Biosciences Pharmingen (Le Pont-de-Claix, France). MSCs were maintained in culture for less than 20 passages in mouse Mesencult stem cell medium (StemCell Technologies, Meylan, France) before being used.
Mice
NOD (H2g7) mice (Taconic Europe, Laven, Denmark) were purchased and bred under specific pathogen-free conditions in our animal facility. Spontaneous diabetes starts at 12 weeks of age in females, and the incidence of diabetes in our colony reaches 76% in females and 15% in males by 30 weeks of age. Diabetes was assessed by measuring urine glucose with chemstrips (Bayer Diagnostics, Puteaux, France). C57BL/6 (H2b, MSC-matched) mice were purchased from Charles River (L’Arbresle, France). The institutional review committee for animal experiments approved all the procedures for mouse care and animal killing.
Effects of MSCs on spontaneous and accelerated diabetes
To examine the protective effects of MSCs against spontaneous diabetes, a single injection of 105 MSCs was administered either i.p. or i.v. to 4-week-old NOD female mice; sham-injected mice served as controls. Accelerated diabetes experiments were performed as previously described, using the adult adoptive cell transfer model [15, 16]. Briefly, splenic CD3+ T cells from diabetic NOD female mice were purified using enrichment columns (R&D Systems Europe, Lille, France) and 5 × 106 T cells from diabetic mice were co-injected with 103 to 106 MSCs i.v. into 8- to 12-week-old irradiated (7.5 Gy) male NOD mice. Control mice were injected with T cells alone. At the end of each experiment, the levels of insulitis of five non-diabetic mice from each group were evaluated, as previously described [15, 16], using 5 µm frozen sections of pancreas as follows: 0, no infiltration; 1, peri-islet infiltration; 2, intra-islet infiltration <50%; 3, extensive infiltration (>50%). After scoring, a mean was calculated.
Flow cytometry analysis
Single cell suspensions were obtained from the spleen and pancreatic lymph nodes (PLNs) of each treated animal as previously described [16]. CD4+ and CD8+ cells were identified in the different experimental groups using appropriate fluorescent conjugated antibodies. To study cellular homing in vivo, either T cells or MSCs (107 cells/ml) were labelled for 10 min at 37°C with 10 µmol/l 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR, USA) immediately before injection, as previously described [16]. CFSE-positive T cells in the thymus, spleen and PLNs at 3, 5 and 7 days after transfer were analysed by flow cytometry. The percentage of labelled cells was evaluated 1, 3, 5 and 7 days after cell transfer. At least 5 × 105 cells were analysed for each sample.
Mononuclear cell culture and proliferation assays
To examine the suppressive capacities of MSCs in vitro, we set up a series of allogeneic mixed lymphocyte cultures. MNCs for proliferation assays were obtained from the spleen of either C57BL/6 (H2b) mice or NOD (H2g7) mice. Cells were proliferated in round-bottomed 96 well plates (Dominique Dutscher, Brumath, France) in a total volume of 0.2 ml RPMI 1640 supplemented with 2 mmol/l glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and 10% (vol./vol.) heat-inactivated fetal calf serum (Invitrogen, Cergy-Pontoise, France). Spleen cells (2 × 105/well) from NOD mice (H2g7) were used as responder cells and primed with irradiated C57/BL6 (H2b) spleen cells (5 × 105/well). Cells harvested at day 7 from these primary cultures were then seeded at a concentration of 2 × 105/well together with increasing numbers (102, 103, 104 per well) of irradiated (60 Gy) MSCs (H2b) and irradiated C57/BL6 splenocytes (2 × 105/well) as antigen-presenting cells (APCs). In some experiments, 100 U/ml IL-2 or 100 U/ml IFN-γ was added to cell cultures to test whether suppression corresponded with T cell anergy or defects in MHC Class II expression. Under each set of conditions, viability was assessed by the Trypan Blue exclusion test. A total of 0.0185 MBq (0.5 µCi) [3H]thymidine (PerkinElmer, Courtaboeuf, France) was added to each well 3 days later, and cells were harvested onto glass fibre Filtermat filters (Skatron Instruments, Lier, Norway) using a 96 well cell harvester (Titertek, Skatron Instruments) after an additional 24 h. A Packard Tricarb beta counter (Perkin-Elmer) was used to measure [3H]thymidine uptake. The results were expressed as the mean cpm of sextuplicates (SEs were routinely <10%).
To test whether MSCs could induce cell apoptosis, we determined indoleamine 2,3-dioxygenase (IDO) activity by measuring the conversion of tryptophan to kynurenine in culture supernatant fractions. We examined several culture conditions, including MSCs cultured alone, which were treated with IFN-γ or untreated, and MSCs co-cultured with MNCs. Tryptophan and kynurenine concentrations were determined by HPLC by H. Faure (Department of Integrative Biology, Centre hospitalier universitaire Michalon, Grenoble, France).
Effects of MSCs on antigen-specific T cell proliferation
Insulin B9–23-specific T cell lines were generated from male NOD mice. Animals were immunised subcutaneously with 100 µg insulin B9–23 peptide (amino acids 9–23 of the B chain; SHLVEALYLVCGERG) [17, 18] in complete Freund’s adjuvant, together with 140 µg of a helper peptide consisting of amino acids 128–140 of hepatitis B virus core antigen (TPPAYRPPNAPIL), at the base of the tail. After 12–14 days, an i.p. boost was given at half dose in incomplete Freund’s adjuvant without helper peptide. After another 12–14 days, mice were killed, then splenocytes were collected and pulsed for 3 h with 10 µmol/l of the same insulin peptide. Splenocytes were then washed, plated and cultured for 6–7 days with 5 U/ml recombinant human IL-2. T cell line specificity was tested with an in-house IFN-γ enzyme-linked immunoassay in the presence of fresh splenocytes pulsed with either insulin B9–23 peptide or DMSO.
For MSC suppression assays, male NOD splenocytes pulsed with 10 µmol/l insulin B9–23 peptide and irradiated at 35 Gy were used as APCs and plated at a concentration of 150,000 cells/well in 96 well round-bottomed plates. T cell lines were added at a concentration of 75,000 cells/well, together with different numbers of irradiated MSCs. After 24 h, 50 µl of the supernatant fractions were removed from each well and concentrations of IL-4, IL-10, IFN-γ were measured by a mouse Th1/Th2 ELISA Ready-SET-GO! Kit (eBiosciences, Clinisciences, Montrouge, France). After an additional 48 h, [3H]thymidine was added overnight and cells from each plate were harvested and counted the following morning.
To study the effects of MSCs on insulin-specific T cell responses, we performed CFSE dilution assays using flow cytometry. An optimal ratio of one MSC to 250 CFSE-labelled T cells was used in these experiments. To study the induction of regulatory T cells, cultures were set up as described above using CFSE-labelled T cell lines and CD4-depleted irradiated splenocytes, either in the presence or absence of 1B1 anti-IL10 receptor-blocking monoclonal antibody (10 µg/ml; kindly provided by A. Lehuen, INSERM U561, Paris, France). After 96 h, brefeldin A (5 µg/ml; Sigma, St Quentin Fallavier, France) was added overnight, after which time cells were harvested, stained with anti-CD4-PerCP-Cy5.5, anti-IL-10-R-PE (both from BD Biosciences Pharmingen) and anti-FOXP3-APC (Miltenyi Biotec, Paris, France) monoclonal antibodies and analysed on a BD FACSAria flow cytometer (BD Biosciences).
FOXP3 analysis
Spleens were collected from male NOD mice, 5 days after transfer of 5 × 106 T cells, either alone or with 106 MSCs, as well as from untreated mice. Purified T cells were examined by flow cytometry to evaluate CD4 and FOXP3 expression. Total RNA was isolated from another set of T lymphocytes from the same experimental mice using the Trizol reagent method (Invitrogen). Total RNA (1 µg) was reverse-transcribed in a final volume of 20 µl containing 100 U Superscript II (Invitrogen), using 0.5 µg random hexamer primers and 0.5 µg oligo(dT)15 primer (Promega, Charbonnières, France), 6.25 mmol/l dNTP (Promega), 0.1 mol/l dithiothreitol and 5′ First-Strand Buffer (Invitrogen). The resultant cDNA was treated with 5000 U/ml RNAse-H (New England BioLabs/Ozyme, Saint Quentin en Yvelines, France). Real-time RT-PCR using a LightCycler (Roche Diagnostics, Meylan, France) was performed in a final volume of 20 µl containing 15 µl of reaction buffer from the FastStart DNA Master Plus SYBR Green I kit (Roche Diagnostics), 10.5 pmol of each forward and reverse specific primer (Eurobio, Les Ulis, France) and 5 µl of a 60-fold dilution of the reverse transcription product. Pairs of PCR primers were designed using the Beacon Designer software (http://beaconsoftware.net/, last accessed 8 April 2009), as follows. Foxp3 primer sequences: Forward: 5′-CCCAGGAAAGACAGCAACCTT-3′, Reverse: 5′-TTCTCACAACCAGGCCACTTG-3′. Samples were quantified using the LightCycler relative quantification software (Roche Diagnostics). Specific mRNA levels were expressed as a percentage relative to the level of mouse Hprt1 mRNA.
Statistical analysis
Cell transfers were analysed by comparison of survival curves using Wilcoxon’s rank-sum test. Score of insulitis, cell proliferation, cytokine analysis and Foxp3 expression data were compared using the Student’s t test for unpaired samples. A p value of less than 5% was considered statistically significant.
Results
MSCs protect NOD mice from diabetes
MSCs from BALB.B (H2b) mice constitutively expressed CD105, CD73, CD29, CD44 and MHC class I molecules, but not CD14, CD34, CD45 (haematopoietic markers), CD31 (endothelial marker) or MHC class II molecules (Electronic supplementary material [ESM] Fig. 1). The same cells exhibited multilineage differentiation potential as assessed by culturing in adipogenic medium (not shown).
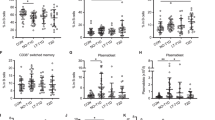
To examine the protective effect of MSCs, we first conducted a series of co-transfer experiments that involved the co-injection of 5 × 106 diabetogenic T cells and an increasing number of MSCs (103 to 106) into irradiated NOD male mice. As a control, a series of 43 irradiated male NOD mice were only injected with 5 × 106 T cells from diabetic mice. The results from five independent experiments are presented in Fig. 1. While the co-injection of 103 or 104 MSCs together with 5 × 106 diabetogenic T cells had no significant effect on diabetes prevalence (9/11 [81.2%] or 15/21 [71.4%] vs 42/43 [97.7%] for mice injected with 5 × 106 diabetogenic T cells alone, p = NS), higher concentrations of MSCs (105 or 106 per mouse) delayed the onset of diabetes and decreased the incidence of diabetes 34 days after cell transfer (6/11 [54.5%] and 2/16 [12.5%] diabetic mice, respectively, p < 0.01 and p < 0.001). As shown in Fig. 2, histological analysis of the pancreases of the mice at 34 days after cell transfer revealed that mice injected with MSCs showed a marked reduction in inflammation compared with those injected with diabetogenic T cells alone, and that the percentage of intact islets was higher in mice injected with 106 MSCs than in those injected with lower levels. The mice injected with diabetogenic T cells alone had significant insulitis (i.e. stage 2–3) in 90% of islets. When co-injected intravenously with 5 × 106 diabetogenic T cells into irradiated NOD male mice, splenic T cells (5 × 106) from protected animals did not exert a protective effect (data not shown).
MSCs confer protection against the transfer of diabetes by NOD splenic T cells. Splenic T cells (5 × 106) from NOD diabetic female mice were intravenously injected into irradiated syngeneic male recipients, either alone (white squares, n = 43) or together with (black symbols) 103 (circles, n = 11), 104 (triangles, n = 21), 105 (diamonds, n = 11) or 106 (squares, n = 15) MSCs. Mice were subsequently followed to determine diabetes development. The results are the means of five independent experiments
MSC administration reduces the severity of insulitis. Diabetogenic NOD spleen cells (5 × 106) were intravenously injected into irradiated syngeneic male recipients, either alone or together with 104, 105 or 106 MSCs. Non-diabetic mice were killed 34 days after cell transfer and the insulitis level scored on pancreatic sections. White fill, stage 0, no infiltration; grey fill, stage 1, peri-islet infiltration; hatched fill, stage 2, intra-islet infiltration <50%; black fill, stage 3, extensive infiltration (≥50%). A mean score was calculated from five mice per group
To examine the effect of MSCs on the incidence of spontaneous diabetes, we treated 4-week-old female mice with a single i.p. or i.v. injection of 105 MSCs. As shown in Fig. 3, the injection delayed the spontaneous onset of diabetes and decreased the incidence of the disease, with 16 out of 27 (59.3%) MSC-treated mice vs 26 out of 26 (100%) control animals becoming diabetic at 36 weeks of age (p < 0.05).
MSCs protect female NOD mice from spontaneous diabetes. A single injection of 105 MSCs was administered either intraperitoneally (black circles, n = 14) or intravenously (squares, n = 13) to 4-week-old NOD female mice. The control mice were injected with PBS alone (open squares, n = 26). Animals were subsequently followed to determine diabetes development
Migration of CFSE-labelled cells
To examine whether MSCs modify T cell homing to the pancreas and lymphoid organs, we performed a series of co-transfer experiments using CFSE-labelled, intravenously injected T cells to follow their migration in irradiated recipients (ESM Fig. 2 and Table 1). Although MSC-injected mice displayed tendencies towards a decrease in the number of diabetogenic T cells in the spleen and an increase in the number in PLNs as compared with mice that received T cells alone, these differences were not statistically significant. We therefore conclude that the MSCs did not interfere with T cell homing.
Next, we followed the migration of MSCs in recipient mice, using CFSE-labelled i.v. injected MSCs (ESM Fig. 2). As illustrated in Fig. 4, the concentration of MSCs was significantly higher in the PLNs than in the spleen, with peak values achieved at day 5 (4.40 ± 0.72% vs 1.86 ± 0.14%, respectively, p < 0.01). Interestingly, these differences persisted 18 days later (1.75 ± 0.25% vs 0.52 ± 0.04%, respectively, p < 0.01). However, we were not able to detect any CFSE-labelled MSCs in the islets of recipient mice by direct immunofluorescence analysis of pancreatic sections from recipient mice at 5, 11 and 18 days after cell transfer (data not shown).
Injected MSCs preferentially home to PLNs. CFSE-labelled MSCs were co-transferred with 5 × 106 diabetogenic T cells into irradiated male NOD recipients. Mice were subsequently killed at the indicated time points, and the number of CFSE+ MSCs in the spleen (white bars) and PLNs (black bars) were determined by flow cytometry. The graph shows the percentage of CFSE+ MSCs injected that were detected in the spleen and PLNs
MSCs inhibit alloreactive NOD T cells in secondary H2-mismatched mixed lymphocyte reactions
To test their ability to inhibit alloreactivity, MSCs were added to secondary allogeneic mixed lymphocyte reactions. As few as 102 MSCs were able to completely suppress alloreactive T cell proliferation (Fig. 5). The addition of exogenous IL-2 to cell cultures did not rescue T cells from MSC suppression (Fig. 5). Moreover, induction of MHC Class II expression by pre-treating MSCs with IFN-γ (ESM Fig. 1) prior to secondary mixed lymphocyte reactions did not result in the recovery of T cell proliferation (data not shown), which suggests that the defect in MHC class II expression cannot account for MSC suppression.
MSCs suppress the proliferation of alloreactive NOD T cells. Increasing numbers of MSCs were added to secondary allogeneic mixed lymphocyte reactions. The graph shows [3H]thymidine incorporation over 25 h. Experiments were performed either in the absence (grey bars) or the presence (open bars) of IL-2. The results are the mean ± SD of three independent experiments
Furthermore, no IDO activity was detected under any of the mixed lymphocyte reaction co-culture conditions; the kynurenine level was <0.6 mmol/l and the tryptophan level was 4.9 mmol/l in all supernatant fractions, compared with 5.9 mmol/l and <0.6 mmol/l, respectively, in the supernatant fractions of positive controls. MSC suppression was not related to the subsequent reduction of cell number and viability measured by the Trypan Blue exclusion method.
MSCs suppress beta cell-specific T cell responses and induce IL-10-producing regulatory T cells in vitro
Finally, we tested whether the MSCs could also suppress T cell responses relevant to autoimmune diabetes in vitro. We chose to study responses against the key epitope, insulin B9–23, as it has been shown to play a critical, and likely initiating, role in the autoimmune beta cell destruction [18]. As previously observed for alloreactive T cell responses, NOD insulin B9–23-specific proliferative responses were significantly suppressed by MSCs in a dose-dependent fashion, from 75% with 102 MSCs to 100% with 105 MSCs (Fig. 6a). As shown in Fig. 6b, this suppression was associated with a dose-dependent increase in IL-10 secretion (p < 0.001 vs no MSCs).
MSCs suppress insulin-specific T cell responses in vitro. a Insulin B9–23-specific T cells were stimulated with B9–23-pulsed irradiated splenocytes in the absence or presence of MSCs. [3H]Thymidine incorporation after a culture period of 72 h is shown. Basal proliferation (i.e. on control-pulsed APCs) was <500 cpm. b The IL-10 level in 24 h culture supernatant fractions was measured by ELISA. All values are expressed as the mean ± SD of measurements made in triplicate. The results in (a) and (b) are representative of three independent experiments. Irradiated splenocyte APCs alone did not display any proliferation or IL-10 secretion (data not shown)
Insulin-specific T cell responses in the presence of MSCs were further investigated by flow cytometry (Fig. 7). Using CFSE dilution assays, we observed the inhibition of T cell proliferation in the presence of MSCs (CD4+CFSE+ T cells: 2.7% vs 0.6% in the absence and presence of MSCs, respectively) (Fig. 7a), and this effect was associated with the induction of IL-10-producing FOXP3+ regulatory T cells (Tregs) (Fig. 7b). Indeed, while some Treg induction was triggered by epitope stimulation alone (2.7% vs 6.4% in the absence and presence of peptide, respectively), the addition of MSCs to the culture significantly increased the proportion of Tregs. This MSC-mediated Treg induction was not antigen specific (21.3% vs 20.2% in the absence and presence of the insulin B9–23 epitope, respectively). Thus, these results indicate that MSCs are able to promote the generation or expansion of Tregs producing both IL-10 and FOXP3. Importantly, however, MSC-mediated suppression was not IL-10-dependent, as blockade of the IL-10 receptor with a neutralising antibody did not prevent the inhibition of T cell proliferation (2.3% vs 0.5% in the absence and presence of MSCs, respectively) (Fig. 7c).
MSC suppression of insulin-specific T cell responses is associated with induction of IL-10-producing Tregs. a Insulin B9–23 peptide (PI B9–23)-specific proliferation in the absence (upper panels) or presence (lower panels) of MSCs using CFSE-labelled insulin-specific T cells. Cells were gated on the CD4+CFSE+ fraction. b Induction of IL-10+FOXP3+CD4+ T cells. These experiments were conducted under the same conditions as those in panel a, with cells gated on the CD4+CFSE+ fraction. c PI B9–23-specific proliferation in the presence of an anti-IL-10 receptor blocking monoclonal antibody
Tregs are induced in vivo following MSC administration
To explore the possibility that the in vivo immunosuppressive action of MSCs was mediated by activation of Tregs in vivo, we analysed the frequency of CD4+FOXP3+ cells in the spleen of irradiated male NOD mice 5 days after i.v. cell transfer. In mice, at day 5, the frequency of CD4+FOXP3+ lymphocytes in the frequency in mice treated with diabetogenic T cells alone (n = 5) was lower than the frequency in untreated mice (2.9 ± 0.3% vs 4.9 ± 1.2%, p < 0.01), while the co-transfer of MSCs with diabetogenic T cells resulted in the recovery of the proportion of CD4+FOXP3+ cells (a representative experiment is shown on Fig. 8). Foxp3 mRNA was also expressed at significantly higher levels in T lymphocytes isolated from the splenocytes of both untreated NOD mice (n = 5) and mice receiving a mixture of diabetogenic T cell and MSCs (n = 7), compared with the mice that received diabetogenic T cells alone (n = 5) (p < 0.001) (Fig. 9). We also analysed the frequency of CD4+FOXP3+ cells in the PLNs of recipient animals. At day 5, the frequency of CD4+FOXP3+ lymphocytes was lower in mice treated with diabetogenic T cells alone (n = 3) than in untreated mice (p < 0.01; n = 3), but co-transfer of MSCs with diabetogenic T cells resulted in the recovery of the percentage of CD4+FOXP3+ cells in the PLNs (7.6 ± 0.8%; n = 3).
Foxp3 expression levels in T splenocytes by real-time PCR. The addition of MSCs to T lymphocytes from diabetic mice significantly increased the level of Foxp3 expression (p < 0.001). Values are the mean ± SD of relative expression ratio as compared with the Hprt1 reference gene (untreated mice and mice treated with diabetogenic T cells, n = 5; mice treated with diabetogenic T cells and MSCs, n = 7)
Discussion
Although the immunosuppressive capacity of MSCs has been demonstrated in several medical conditions, few studies have evaluated their capacity to inhibit T cell function in autoimmune diseases. MSCs exhibit the intriguing characteristics of being able to escape immune recognition and to inhibit immune responses. In mixed lymphocyte reactions, MSCs suppress T cell proliferation independently of MHC matching between T cells and MSCs [19, 20]. Murine MSCs have been shown to prevent experimental autoimmune encephalomyelitis in mice through the induction of peripheral tolerance against the pathogenic antigen [9, 10]. This protection was effective when the MSCs were administered at the onset of the disease or at the peak of the disease, but not after disease stabilisation. Controversial results were obtained in collagen-induced arthritis, but recent reports have shown that MSC infusion prevented tissue damage and exerted immunomodulatory effects by educating antigen-specific Tregs [11, 12].
In the present study we examined the effects of the administration of a single dose MSCs in NOD mice, a mouse model of human type 1 diabetes. We provide strong evidence that MSCs confer significant protection against both T cell-transferred and spontaneous disease. This therapeutic effect correlates with decreased islet infiltration, MSC homing to the draining PLNs, and in vitro suppression of both allo- and beta cell-specific responses. The induction of FOXP3+CD4+ Tregs secreting IL-10 was also observed in vitro, although IL-10 did not prove to be involved in the suppressive mechanisms. Despite this apparent discrepancy, our results are in line with those of Thornton and Shevach [21] for mouse Tregs, and of Jonuleit et al. [22] for human Tregs. In both models, Tregs were induced and produced both IL-10 and TGFβ, but their suppressive activity was not dependent on either cytokine. Our results are also in line with other studies suggesting that MSC-mediated suppression may be contact-dependent in vitro but cytokine-mediated in vivo [4, 21, 22]. Indeed, this discrepancy between in vitro and in vivo MSC suppression studies is reminiscent of the conundrum regarding the mechanisms of suppression of murine Tregs, which also display distinct behaviours in vitro and in vivo [23]. Our results also highlight important differences between mouse and human MSCs [24, 25]. Glennie et al. [25] reported that human MSC-mediated inhibition induces an unresponsive T cell profile that can be reverted by the addition of exogenous IL-2. Interestingly, IL-2 did not restore T cell proliferation in our experiments, suggesting that the MSCs did not induce classical T cell anergy [25]. Moreover, since the number and viability of the cells were not affected, we conclude that MSCs did not induce T cell apoptosis. One other established mechanism of human MSC-mediated suppression is IDO activity upregulation. IDO activation is induced by IFN-γ, and IDO catalyses the conversion of tryptophan into kynurenine [26, 27]. Activation of IDO thus causes tryptophan depletion, resulting in reduced lymphocyte proliferation [28]. In contrast to previous findings in human MSCs stimulated by IFN-γ [29], we did not detect significant IDO activity in MSC-suppressed mixed lymphocyte reactions, even in the presence of IFN-γ (not shown). Thus, the mechanism of MSC-mediated suppression remains controversial. However, although we do not have direct evidence, our data are compatible with a contact-dependent mechanism of suppression, perhaps mediated by an intermediate mediator linking the MSCs to Treg induction. In this respect, Li et al. recently showed that Treg-inducing dendritic cells can be generated through direct cell-to-cell contact between haematopoietic stem cells and MSCs by activation of the Notch signalling pathway [30]. Whether a similar mechanism is at play in our model requires further investigation.
The administration of a single dose of adult MSCs was sufficient to prevent the onset of diabetes. One possible explanation for this efficacy could be related to the regeneration of pancreatic islets by MSCs; however, we considered this hypothesis unlikely since protection was obtained during the early inflammatory phase of the disease. Moreover, Urban et al. [31] have recently reported in a streptozotocin-induced mouse model of diabetes that MSCs cannot induce pancreas regeneration by themselves, as they require co-injection of bone marrow stem cells. In addition, clinical efficacy in our model was correlated with greatly decreased insulitis scores, further suggesting immune-mediated, rather than regenerative, mechanisms. In a recent paper, Tritt et al. [32] demonstrated that a decrease in the frequency of CD4+ Tregs contributes to the onset of autoimmune diabetes. We therefore measured the expression of FoxP3 on cells in the spleen and PLNs of MSC-treated or untreated mice and found that MSC treatment increased FOXP3 T cell frequency. These results correlate with the in vitro findings showing that the immunosuppressive activity of MSCs is correlated with the induction of FOXP3+ T cells. Following this model of Treg-mediated suppression by MSCs, it was surprising to observe that splenic T cells from protected animals were not able to transfer protection. This discrepancy may be due to the preferential homing of induced Tregs towards PLNs. Indeed, MSCs were found to preferentially home to PLNs rather than the spleen. It is therefore reasonable to hypothesise that MSC-induced Tregs may also preferentially reside in PLNs or in the islets themselves. Supporting this possibility, Tritt et al. [32] reported that Tregs preferentially localise in the pancreatic environment, where they suppress the accumulation and function of effector T cells.
In conclusion, we have demonstrated the therapeutic potential of the administration of a single dose of adult MSCs to prevent the development of type 1 diabetes beta cell autoimmunity. This clinical efficacy correlates with the suppressive effects of MSCs on both allogeneic and autoimmune responses in vitro, and with the induction of Tregs both in vivo and in vitro. MSC suppression does not require MHC matching or any additional immunosuppressive therapy. This may have important clinical implications for type 1 diabetes immune interventions.
Abbreviations
- APC:
-
Antigen-presenting cell
- CFSE:
-
5,6-Carboxyfluorescein diacetate succinimidyl ester
- IDO:
-
Indoleamine 2,3-dioxygenase
- MSC:
-
Mesenchymal stem cell
- PLN:
-
Pancreatic lymph node
- Treg:
-
Regulatory T cell
References
Friedenstein AJP, Petrokova KV (1966) Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol 16:381–390
Barry FP, Murphy JM, English K, Mahon BP (2005) Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Dev 14:252–265
Barry FP, Murphy JM (2004) Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 36:568–584
Nauta AJ, Fibbe WE (2007) Immunomodulatory properties of mesenchymal stem cell. Blood 15:3499–3506
Bartholomew A, Sturgeon C, Siatskas M et al (2002) Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30:42–48
Rasmusson I, Ringden O, Sundberg B, Le Blanc K (2003) Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 76:1208–1213
Le Blanc K, Rasmusson I, Sundberg B et al (2004) Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363:1439–1441
Krampera M, Glennie S, Dyson J et al (2003) Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101:3722–3729
Zappia E, Casazza S, Pedemonte E et al (2005) Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T cell anergy. Blood 106:1755–1761
Zhang J, Li Y, Chen J et al (2005) Human bone marrow stromal treatment improves neurological functional recovery in EAE mice. Exp Neurol 195:16–26
Djouad F, Fritz V, Apparailly F et al (2005) Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor α in collagen-induced arthritis. Arthritis Rheum 52:1595–1603
Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G (2007) Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum 56:1175–1186
Abdi R, Fiorina P, Adra CH, Atkinson M, Sayegh MH (2008) Immunomodulation by mesenchymal stem cells. A potential therapeutic strategy for type 1 diabetes. Diabetes 57:1759–1767
Kikutani H, Makino S (1992) The murine autoimmune diabetes model: NOD and related strains. Adv Immunol 51:285–322
Thivolet C, Bendelac A, Bedossa P, Bach JF, Carnaud C (1991) CD8+ T cell homing to the pancreas in the nonobese diabetic mouse is CD4+ T cell-dependent. J Immunol 146:85–88
Aspord C, Czerkinsky C, Durand A, Stefanutti A, Thivolet C (2002) α4 Integrins and L-selectin differently orchestrate T-cell activity during diabetes prevention following oral administration of CTB-insulin. J Autoimmun 19:223–232
Daniel D, Gill RG, Schoot N, Wegmann D (1995) Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol 25:1056–1062
Nakayama M, Abiru N, Moriyama H et al (2005) Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435:220–223
Di Nicola M, Carlo-Stella C, Magni M et al (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838–3843
Rasmusson I (2006) Immune modulation by mesenchymal stem cells. Exp cell Res 312:2169–2179
Thornton AM, Shevach EM (1998) CD4+CD25+ immunoregulatory T cells suppress polyclonal activation in vitro by inhibiting interleukin 2 production. J Exp Med 188:287–296
Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH (2000) Induction of interleukin 10-producing, non-proliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med 192:1213–1222
Bach JF (2003) Regulatory T cells under scrutiny. Nat Rev Immunol 3:189–198
Klyushenkova E, Mosca JD, Zermetkina V et al (2005) T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci 12:45–57
Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F (2005) Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105:1815–1822
Le Rond S, Gonzalez A, Gonzalez AS, Carosella ED, Rouas-Freiss N (2005) Indoleamine 2,3 dioxygenase and human leucocyte antigen-G inhibit the T-cell alloproliferative response through two independent pathways. Immunology 116:297–307
Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC (2005) Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia 19:1597–1604
Terness P, Bauer TM, Rose L et al (2002) Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med 196:447–457
Frumento G, Rotondo R, Tonetti M, Ferrara GB (2001) T cell proliferation is blocked by indoleamine 2,3-dioxygenase. Transplant Proc 33:428–430
Li YP, Paczesny S, Lauret E et al (2008) Human mesenchymal stem cells license adult CD34+ hematopoietic progenitor cells to differentiate into regulatory dendritic cells through activation of the NOTCH pathway. J Immunol 1:1598–1608
Urban VS, Kiss J, Kovacs J et al (2008) Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells 26:244–253
Tritt M, Sgouroudis E, d’Hennezel E, Albanese A, Piccirillo CA (2008) Functional waning of naturally occurring CD4+ regulatory T cells contributes to the onset of autoimmune diabetes. Diabetes 57:113–123
Acknowledgements
We are indebted to E. Simpson (Department of Immunology and Transplantation Biology, Hammersmith Hospital, London, UK) for the gift of the MSCs, to A. Durand (INSERM U870/INRA 1235/INSA/Université Lyon1/Hospices Civils de Lyon, Lyon, France) for excellent technical assistance and for assistance with the NOD colony, and to J. Plumas (Laboratoire R&R, EFS Rhône-Alpes, Grenoble, France) for analysis of the IDO results. This study was supported by INSERM grants to A. M. Madec and C. Thivolet, and by grants from the European Foundation for the Study of Diabetes (EFSD-JDRF-Novo Nordisk award), the Fondation Recherche Médicale (Installation Nouvelle Equipe) and the Programme National de Recherche sur le Diabète 2007 (to R. Mallone).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
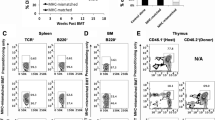
Immunophenotype of MSCs. MSCs from BALB.B (H2b) mice were analysed by FACS for the expression of CD14, CD45, CD105, CD73, CD34, CD31, CD29, CD44 and MHC class I and class II molecules under standard conditions (black fill) or after being cultured for 48 h in the presence of IFNγ for the expression of MHC class II molecules (grey fill). The grey trace (no fill) denotes the isotype control. The bars labelled M1 indicate the antigen-positive cells (PDF 89 kb)
ESM Fig. 2
Migration of CFSE-labelled cells. The frequency of CFSET cells and CFSE MSCs in the spleen and PLNs of irradiated recipients was analysed by flow cytometry 5 days after the co-transfer experiments using either CFSE-labelled T cells or CFSE-labelled MSCs. Plots representative of three independent experiments are shown (PDF 41 kb)
Rights and permissions
About this article
Cite this article
Madec, A.M., Mallone, R., Afonso, G. et al. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia 52, 1391–1399 (2009). https://doi.org/10.1007/s00125-009-1374-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-009-1374-z