Abstract
Aim/hypothesis
Studies investigating the structure, neurophysiology and functional outcomes of white matter among type 1 diabetes patients have given conflicting results. Our aim was to investigate the relationship between type 1 diabetes and white matter hyperintensities.
Method
We assessed white matter integrity (using magnetic resonance imaging), depressive symptoms and neuropsychological function in 114 type 1 diabetes patients and 58 age-matched non-diabetic controls.
Results
Only Fazekas grade 1 and 2 white matter hyperintensities were found among 114 long-duration, relatively young diabetes patients; the severity of lesions did not differ substantially from 58 healthy controls. White matter hyperintensities were not associated with depressive history or with clinical characteristics of diabetes, including retinopathy, severe hypoglycaemia or glycaemia control.
Conclusions/interpretation
Our data do not support an association between diabetes characteristics and white matter hyperintensities among relatively young type 1 diabetes participants.
Similar content being viewed by others
Introduction
Type 1 diabetes leads to microvascular complications in the retina and kidney, and to peripheral and autonomic neuropathy secondary to chronic hyperglycaemia [1], but its impact on the central nervous system remains poorly understood. Neurocognitive research suggests that type 1 diabetes may be associated with psychomotor slowing and reductions in mental efficiency [2, 3]—a pattern consistent with damage to brain white matter. However, studies investigating the impact of type 1 diabetes on white matter structure, neurophysiology and functional outcomes have been equivocal [4–11].
Some researchers have reported periventricular white matter abnormalities [8], subcortical or brain stem lesions [7], small focal white matter hyperintensities in the basal ganglia [4] and/or cortical atrophy [5], whereas others found no evidence of white matter anomalies [6, 10, 11]. These null results were most common in studies that included a non-diabetic comparison group. The presence of microvascular complications has also been associated with white matter abnormalities, although again, there is not complete consensus. Retinopathy was associated with white matter hyperintensities in some [4, 7], but not all reports [9, 11]. In contrast, neither recurrent hypoglycaemia [8], nor early age at onset [5], predicted the occurrence of white matter abnormalities.
In a previous report, we used neuroimaging techniques to estimate the effects of type 1 diabetes on grey matter density [12] and found that high HbA1c levels and a history of severe hypoglycaemic events were associated with lower grey matter density. We have used neuroimaging techniques to examine white matter integrity in a larger sample of young adults with type 1 diabetes of long duration, and in a comparison group of demographically similar non-diabetic adults. We addressed four questions: (1) Do patients with type 1 diabetes differ in the number and severity of white matter hyperintensities compared with age-matched non-diabetic controls? (2) If so, what biomedical markers (lipid profile, blood pressure, HbA1c, retinopathy) are associated with white matter hyperintensities? (3) Does diabetes affect cognition, and if so, are cognitive effects linked to white matter hyperintensities? (4) Given the high rate of depression in diabetic patients, is history of depression associated with white matter hyperintensities or cognitive dysfunction?
Methods
Sample
We recruited patients with type 1 diabetes (duration 15 to 28 years) while non-diabetic controls were recruited from the community and through patient referrals. All were 25 to 40 years old and either right-handed or ambidextrous. Those with painful neuropathy, clinically significant nephropathy, a diagnosis of schizophrenia, bipolar disorder or attention deficit hyperactivity disorder were excluded. Patients were also excluded if they had clinically evident atherosclerotic disease such as coronary artery, peripheral vascular or cerebral vascular disease as manifested by stroke, angina, history of myocardial infraction, intermittent claudication or other manifestations. We intentionally oversampled people with a history of major depression. All potential participants were assessed for depression with initial screening questions and the Structured Clinical Interview for DSM-IV. Once we reached a sufficient sample size of non-depressed participants, we closed that arm of the study to recruitment and continued screening to fill the depressed cohort. No special recruiting was done to seek depressed participants. However, current major depression was exclusionary, as were any contraindication to magnetic resonance imaging (MRI) scanning (e.g. gunshot wound, pacemaker, pregnancy or claustrophobia). The Joslin Diabetes Center Committee on Human Subjects approved the study protocol and each participant provided informed written consent prior to participating. To assess for hypo- or hyperglycaemia, blood glucose levels were measured using finger sticks and glucose meters prior to the MRI and prior to and during each psychosocial and cognitive assessment.
Measures
MRI image acquisition and lesion grading
MRI scanning occurred at the McLean Brain Imaging Center (Belmont, MA, USA) using a 1.5 T GE whole-body imaging system (Horizon LX, General Electric Medical Systems, Milwaukee, WI, USA) and a custom-made linear birdcage coil with approximately 40% improvement in signal-to-noise ratio and improved homogeneity over standard quadrature head coil [13]. A three-dimensional spoiled gradient echo pulse sequence was used to produce 1.5 mm thick contiguous coronal images [n = 124; echo time (TE) = 5 ms, repetition time (TR) = 35 ms, 256 × 192 matrix; field of view (FOV) = 24 cm, flip angle = 45°, 1 number of excitations (NEX)], as well as fluid attenuated inversion recovery (FLAIR) axial images (TE = 133 ms, TR = 9000 ms, TI = 2200 ms, matrix 256 × 192, FOV = 20 cm, NEX = 1, 5 mm slice thickness, 2.0 mm slice gap, flip = 90°). Axial proton-density and T-2 weighted images (TE = 30/80 ms, TR = 3000 ms, 256 × 192 matrix; FOV = 20 cm, flip angle = 90°, 0.5 NEX, 3 mm thick slices, no skip) were obtained to screen for brain structural abnormalities.
Foci of hyperintensity in the deep white matter were rated using the Fazekas classification system [14]: grade 0 = absence; grade 1 = punctuate foci; grade 2 = beginning confluence of foci; grade 3 = large confluent area. Participants with grade 1 white matter hyperintensities by the Fazekas classification were then subclassified as follows according to the modified version of the Coffey classification [15, 16]: grade 1–1 = (n = 1 or 2 and each with a diameter < 5 mm); grade 1–2 = (n < 10 and the largest lesion having a diameter 5–10 mm); grade 1–3 = (n ≥ 10 or at least one lesion with a diameter > 10 mm).
For periventricular white matter hyperintensities, grading also used the Fazekas classification system [14]: grade 0 = absence; grade 1 = ‘cap’ or pencil-thin lining, grade 2 = smooth ‘halo’, grade 3 = irregular periventricular lesions extending into the deep white matter. Two experienced neuroradiologists, blind to participants’ clinical status, separately reviewed T2 and FLAIR images to determine prevalence, severity and location of deep and periventricular white matter hyperintensities (weighted κ statistics ranged from 0.93 to 0.97; Shrout–Fleiss intraclass correlations ranged from 0.93 to 0.98).
Cognitive assessment
Attention, memory, problem-solving and executive function, mental and motor efficiency, and spatial skills were assessed with a battery of well-known measures: the Wechsler Abbreviated Scale of Intelligence (Similarities, Block Design, Vocabulary and Matrix Reasoning subtests) [17]; Digit Symbol Substitution [18]; Sorting, Color–Word Inhibition, Trail Making Number–Letter Switching, Design Fluency and Letter Fluency tasks from the Delis–Kaplan Executive Function System [19]; the Letter–Number Sequencing, Spatial Span and Verbal Paired Associate tasks (I and II) from the Wechsler Memory Scale [20]; and the Grooved Pegboard [21].
Medical history data
Medical data were obtained through review of medical records and patient self-reported health history. Retinopathy status was obtained from reports of dilated retinal examinations done at the Joslin Diabetes Center Betham Eye Institute by ophthalmologists who specialise in the treatment of people with diabetes and who are certified for national clinical trials in the Diabetic Retinopathy Clinical Research Network. Diabetes participants reported lifetime number of severe hypoglycaemic events leading to unconsciousness [1].
Lifetime average metabolic control was estimated by averaging all available HbA1c values; results were grouped in 4 year increments, beginning with the first year of diagnosis and working to the present (HPLC ion capture method; Tosch Medics, San Francisco, CA, USA; reference range 4.0–6.0%). For earlier assessments that used HbA1 to assess glycaemic control, we converted HbA1 values to HbA1c values using the formula HbA1c = (HbA1–0.14)/1.21 [22]. Hand preference was determined with the Edinburgh Handedness Inventory [23].
Psychiatric history and symptom
The Structured Clinical Interview for DSM-IV (SCID) [24, 25] was used to ascertain past and current psychiatric diagnoses; the Hamilton-D scale [26] and the Symptoms Checklist-90-R (SCL90-R) [27] provided self-report information on current psychiatric symptomology. Participants with substance dependence and those who scored higher than 14 on the Hamilton-D scale [26] were excluded.
Data analyses
Student’s and non-parametric t tests were used to compare group differences in continuous demographic, and clinical and cognitive data, while Fisher’s exact test, χ 2 test and dichotomous logistic regression were used to assess between-group differences in presence of white matter hyperintensities (whether or not any grade of lesions was present). We used ordinal (proportional odds model) logistic regression to assess group differences in the severity of deep and periventricular white matter hyperintensities compared with having no white matter hyperintensity lesion. Only one person had grade 1–3 deep white matter hyperintensities; thus the odds ratio for grade 1–3 vs no lesion could not be estimated. Analyses used SAS Statistical Software (version 8.2; SAS Institute, Cary, NC, USA). For analysis of cognitive data, we used analysis of covariance to adjust for depressive symptoms. To assess the strength of associations of severe hypoglycaemic events on brain structure, we divided patients into two groups: (1) no severe hypoglycaemic events; and (2) one or more hypoglycaemic event according to the strict DCCT criteria in which the event leads to coma or unconsciousness [1, 28]. Data are presented as mean ± SD or as percentages. Because of the number of statistical tests, the α level was set to ≤0.01, two-tailed.
Results
The study sample included 114 type 1 diabetes patients and 58 age-matched non-diabetic control participants (Table 1). Participants were young (range 25 to 40 years old), well-educated and healthy. HbA1c for diabetic participants was 7.8% (range 5.3 to 14.1%) and 5.1% (range 4.3 to 5.8%) for control participants. Although diabetic participants had higher systolic blood pressure (p < 0.01), blood pressure values for both groups were, on average, within normal targets (120/80 mmHg). Because we oversampled for those with a history of major depression, a large portion of both groups had a past diagnosis of major depressive disorder (36% for the control group and 32% for the diabetes group; NS). Although diabetic participants had somewhat higher scores on the SCL90-R depression subscale (p = 0.003), the mean SCL90-R score for both groups was <63, the typical cut-off for screening depression. Retinopathy data was available on 88 diabetic participants: 39% had no retinopathy, 46% had non-proliferative retinopathy and 16% had proliferative retinopathy.
White matter hyperintensities
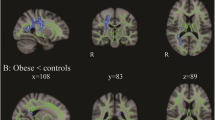
Both diabetic (31%) and control participants (40%) had mild deep white matter hyperintensities (grades 1–1 and 1–2). One person with diabetes had grade 1–3 hyperintensities. No one had grade 2 lesions in deep white matter or grade 3 lesions in either deep or periventricular white matter. The groups did not differ on proportion of participants with deep white matter (based on scoring that accounted for number and size of lesions) or with periventricular white matter hyperintensities (Table 2). Figure 1a shows an image with grade 1–2 lesions in deep white matter while Fig. 1b shows a typical image with grade 2 periventricular lesions. Logistic regression found that demographic characteristics, smoking, systolic or diastolic blood pressure, triacylglycerol, meeting HDL- or LDL-cholesterol target levels, and being on lipid-lowering or antihypertensive medications were not associated with the presence or severity of deep or periventricular white matter hyperintensities in this sample. We found a very modest relationship between having any periventricular hyperintensity lesion and diabetes (odds ratio = 2.3, 95% CI 1.02–5.23; p = 0.04). However, when we adjusted for smoking (odds ratio = 2.16, 95% CI 0.94–5.0), ethnicity, being on lipid-lowering medications, or blood pressure medications individually (data not shown) or as a group, the model no longer reached statistical significance (Table 2). Meeting HDL- or LDL-cholesterol targets was associated with the presence of deep white matter hyperintensities. Interestingly, 68% of the non-diabetic and 76% of the diabetic groups were at desired HDL-cholesterol target levels or higher, while only 35% of those with diabetes and 44% of controls met LDL-cholesterol targets. Within-group analyses showed no effects of age at diagnosis, history of severe hypoglycaemia, presence of retinopathy (neither background nor severe) or lifetime HbA1c on the number and severity of deep white matter or on the severity periventricular white matter hyperintensities.
Cognition and white matter hyperintensities
Participants scored within the normal range in all cognitive domains, but diabetic participants scored lower than controls on vocabulary, one measure of executive function (Sorting Test), short-term memory, psychomotor efficiency, one measure of mental efficiency and delayed recall (Table 3). No statistically significant differences existed between the two groups on spatial ability, attention, working memory, or on three of the four mental efficiency tasks. The odds for better performance on one delayed memory measure (Verbal Paired Associates II) were greater for those without periventricular white matter hyperintensities compared with those with periventricular hyperintensities (per one unit difference, odds ratio = 1.2, 95% CI = 1.06–1.38; p = 0.006); the association remained after controlling for depression, demographic and other variables, but fell into the trend range after controlling for diabetes (odds ratio = 1.2, 95% CI = 1.02–1.43; p = 0.02). No other associations were noted between performance on other cognitive tasks and periventricular or deep white matter hyperintensities. Retinopathy severity was not associated with performance on cognitive tests. Depressive symptoms were, however, modestly correlated with poorer neuropsychological performance among the total sample (Table 3). After adjusting for depressive symptoms, between group differences in cognitive scores remained essentially the same.
Depressive symptoms and white matter hyperintensities
A history of major depression was not associated with the presence or severity of white matter hyperintensities in either diabetic or non-diabetic participants (NS). A trend for a relationship between current subclinical depressive symptoms and presence of deep white matter hyperintensities existed within the non-diabetic group (for a ten point difference in SCL90 depression score, odds ratio = 1.85, 95% CI = 0 1.09–3.14) but not within the diabetic group. Depressive symptoms were modestly correlated with poorer neuropsychological performance among the total sample (Table 3).
Discussion
Our sample of young adults with type 1 diabetes (mean age 32 ± 4 years) and long duration diabetes (mean duration 20 ± 4 years) showed evidence of only mild white matter hyperintensities (grades 1 and 2), which were comparable to those seen in a demographically similar sample of non-diabetic adults. These findings are consistent with those of another study [6] that obtained MRI and cognitive measures from 40 somewhat older diabetic participants and 40 age-matched non-diabetic comparison participants. Another study also reported null results in an investigation of 25 type 1 diabetic patients who were compared with ten non-diabetic controls [11]. In contrast, others [7] reported that 69% (11 of 16) patients with long duration diabetes vs 12.5% of 40 non-diabetic controls had white matter hyperintensities ranging from 2 to 10 mm (mean 5 mm) and that seven of these 11 patients had lesions ≤ 5 mm. These authors did not find a relationship with arterial blood pressure, but did not report how many patients had hypertensive disease. All patients had peripheral neuropathy and many had other complications including autonomic neuropathy and nephropathy. Other cardiovascular risk factors, such as lipid levels, were not reported. On the other hand, control participants were free of cardiovascular risk factors, thus the impact of comorbidities cannot be separated from that of hyperglycaemia alone.
The number and severity of lesions for type 1 diabetes patients in our study are consistent with data from Brands [6], who found that most diabetic patients and controls had some white matter lesions, and the severity and prevalence did not differ between groups. Studies examining white matter lesions in non-diabetic, asymptomatic volunteers aged 20 to 83 years found that 11 to 73% of participants had white matter hyperintensities ranging from grade 1 to grade 3 [29–34] with more white matter hyperintensities occurring among older participants [29, 32]; for those under 50 years of age, 11 to 42% of participants had either deep white matter or periventricular hyperintensities [29, 31, 33, 34]. This prevalence of white matter hyperintensities in non-diabetic controls suggests that some white matter hyperintensities, particularly grades 1 and 2, may not be clinically significant and emphasises the importance of including a non-diabetic control group when assessing the effect of diabetes on brain structure.
Whether periventricular grade 1 and 2 white matter hyperintensities are precursors to more severe lesions remains unclear. A study examining histopathological changes among 11 older people who underwent MRI prior to death found that all grades of deep white matter hyperintensities appeared to be associated with increasing severity of ischaemic tissue damage and marked arteriosclerosis while periventricular grades 1 and 2 appeared to be non-ischaemic in origin [35]. A similar histopathology study replicated these findings [36]. In our study, HDL-cholesterol levels were approximately within normal limits, although LDL-cholesterol levels were, on average, above desired targets of 2.59 mmol/l (http://www.joslin.org/managing_your_diabetes_joslin_clinical_guidelines.asp). HDL-cholesterol levels for total participants, but not other markers of dyslipidaemia, appeared to offer a small measure of protection from deep white matter hyperintensities, as those with higher HDL-cholesterol levels were less likely to have deep white matter hyperintensities. If deep white matter and grade 3 periventricular lesions are related to ischaemia and arteriosclerotic disease [35, 36], these characteristics combined with normal ageing may help explain differences on MRI studies between people with type 1 and type 2 diabetes.
Of the studies that assessed smoking, some but not all studies have found that smoking history is associated with increased white matter hyperintensities, particularly in older people [37–40]. These studies typically used a crude scale (never smoked, former smoker, current smoker) with approximately 50% having a history of smoking. We used pack-years in our study and when we examined the percentage of individuals who had never smoked, 75% of those with diabetes and 87% of controls never smoked, thus explaining the very low pack-years rate in our relatively young cohort. The diabetic group smoked, on average, for 1.3 pack-years vs 0.2 pack-years for controls; although statistically different, this amount of smoking may not be enough to clinically impact white matter integrity, as fewer of the diabetic patients had deep white matter lesions compared with controls. When the deep white matter hyperintensity analysis was adjusted for smoking, the p value for having diabetes remained non-significant. When the periventricular hyperintensity model was adjusted for smoking, the p value for the impact of diabetes moved from a trend (p = 0.04) to non-significant (p = 0.07).
Studies have also examined whether hypoglycaemia and/or age of onset contribute to the formation of white matter hyperintensities. Two studies [4, 8] examining the impact of a history of severe hypoglycaemia found no impact on the number or severity of white matter hyperintensities [4]; in one study, however, participants with a hypoglycaemic history had more cortical atrophy [8]. Ferguson et al. [5] reported that those with early onset diabetes (<7 years of age) did not differ from those with later onset diabetes (>7 years of age) in terms of white matter hyperintensities, although the younger onset group had more prevalent ventricular atrophy and greater lateral ventricular volumes. Our findings on white matter hyperintensities are consistent with those in these previous publications.
This same investigator group [4] also found that 25 type 1 diabetes patients with background retinopathy had more small focal white matter hyperintensities than 46 people without retinopathy while, in our study, no differences existed based on the presence of background or proliferative retinopathy. Others [11] found no relationship between proliferative retinopathy and white matter lesions. Diabetic retinopathy reflects the development and progression of microvascular disease associated with chronic hyperglycaemias. Thus, the lack of association between white matter hyperintensities and retinopathy in our data suggests that early white matter hyperintensities may result from causes other than diabetes related microvascular disease.
Diabetic participants performed somewhat worse than control participants on psychomotor efficiency, some verbal memory tasks and on a card-sorting task requiring organisational and conceptual skills. Effect sizes ranged from 0.3 to 0.6 and are comparable to those reported by others [3]. Consistent with our study results, lower grooved pegboard scores are frequently reported in studies of adults with type 1 or type 2 diabetes—particularly with clinically significant complications [2]. The lack of difference in white matter hyperintensities may help explain why between-group differences in psychomotor speed were relatively small.
In our prior report on a subset of this sample [12], we examined grey matter and found that diabetic patients showed lower grey matter density than controls and that lower grey matter density was associated with high HbA1c levels and severe hypoglycaemic events. This finding, coupled with our current data, suggests that cognitive problems, if present, may be due largely to changes in grey matter rather than in white matter in people with type 1 diabetes.
As noted in the methods section, to obtain a sufficiently large sample of participants with history of depression, we used selective sampling by continuing to screen for a history of depression without current depression. This sampling scheme has the potential of affecting the make-up of the groups and confounding the between-group analysis of white matter hyperintensities. However, white matter hyperintensities and history of depression were clearly not related, so it is unlikely that our sampling system had any influence on our primary results.
In summary, we found a similar prevalence of grade 1 and 2 white matter hyperintensities in young adults with or without diabetes mellitus. These white matter hyperintensities were not associated with depressive history or with clinical characteristics of diabetes including retinopathy, history of severe hypoglycaemia or lifetime glycaemic control. Moreover, these were not robustly associated with the cognitive test scores of either people with diabetes or in age-matched controls. It is possible that newer, more sensitive imaging techniques may find subtle differences that MRI techniques used in this study could not.
Abbreviations
- FLAIR:
-
fluid attenuated inversion recovery
- FOV:
-
field of view
- MRI:
-
magnetic resonance imaging
- NEX:
-
number of excitations
- TE:
-
echo time
- TR:
-
repetition time
References
DCCT (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329:977–986
Ryan CM, Geckle MO, Orchard TJ (2003) Cognitive efficiency declines over time in adults with type 1 diabetes: effects of micro- and macrovascular complications. Diabetologia 46:940–948
Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP (2005) The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care 28:726–735
Ferguson SC, Blane A, Perros P et al (2003) Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes 52:149–156
Ferguson SC, Blane A, Wardlaw J et al (2005) Influence of an early-onset age of type 1 diabetes on cerebral structure and cognitive function. Diabetes Care 28:1431–1437
Brands AM, Kessels RP, Hoogma RP et al (2006) Cognitive performance, psychological well-being, and brain magnetic resonance imaging in older patients with type 1 diabetes. Diabetes 55:1800–1806
Dejgaard A, Gade A, Larsson H, Balle V, Parving A, Parving HH (1991) Evidence for diabetic encephalopathy. Diabet Med 8:162–167
Perros P, Deary IJ, Sellar RJ, Best JJ, Frier BM (1997) Brain abnormalities demonstrated by magnetic resonance imaging in adult IDDM patients with and without a history of recurrent severe hypoglycemia. Diabetes Care 20:1013–1018
Lunetta M, Damanti AR, Fabbri G, Lombardo M, Di Mauro M, Mughini L (1994) Evidence by magnetic resonance imaging of cerebral alterations of atrophy type in young insulin-dependent diabetic patients. J Endocrinol Invest 17:241–245
Lobnig BM, Kromeke O, Optenhostert-Porst C, Wolf OT (2006) Hippocampal volume and cognitive performance in long-standing Type 1 diabetic patients without macrovascular complications. Diabet Med 23:32–39
Yousem DM, Tasman WS, Grossman RI (1991) Proliferative retinopathy: absence of white matter lesions at MR imaging. Radiology 179:229–230
Musen G, Lyoo IK, Sparks CR et al (2006) Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes 55:326–333
Dager SR Friedman SD, Parow A, Demopulos C et al (2004) Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry 61:450–458
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149:351–356
Bae SC, Lyoo IK, Sung YH et al (2006) Increased white matter hyperintensities in male methamphetamine abusers. Drug Alcohol Dependence 81:83–88
Lyoo IK, Streeter CC, Ahn KH et al (2004) White matter hyperintensities in subjects with cocaine and opiate dependence and healthy comparison subjects. Psychiatry Res 131:135–145
Wechsler D (1999) Wechsler Abbreviated Scale of Intelligence manual. The Psychological Corporation, San Antonio, TX
Wechsler D (1997) WAIS-III Wechsler Adult Intelligence Scale, 3rd edn. The Psychological Corporation, San Antonio, TX
Delis DC, Kaplan E, Kramer JH (2001) The Delis–Kaplan Executive Function System: examiner’s manual. The Psychological Corporation, San Antonio, TX
Wechsler D (1997) Wechsler Memory Scale, 3rd edn. The Psychological Corporation, San Antonio, TX
Matthews CG, Klove H (1964) Instruction manual for the Adult Neuropsychology Test Battery. University of Madison Medical School, Madison, WI
Krolewski AS, Laffel LM, Krolewski M, Quinn M, Warram JH (1995) Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med 332:1251–1255
Oldfield R (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Spitzer RL, Williams JB, Gibbon M, First MB (1992) The structured clinical interview for DSM-III-R (SCID). I: History, rationale and description. Arch Gen Psychiatry 49:624–629
Williams JB, Gibbon M, First MB et al (1992) The Structured Clinical Interview for DSM-III-R (SCID). II. Multisite test–retest reliability. Arch Gen Psychiatry 49:630–636
Hamilton M (2000) Handbook of psychiatric measures. The American Psychiatric Association, Washington, DC
Derogatis LR (1994) SCL-90-R: Symptom Checklist-90-R. Administration, scoring and procedures manual. National Computer Systems, Minneapolis, MN
CCT (1996) Effects of intensive diabetes therapy on neuropsychological function in adults in the Diabetes Control and Complications Trial. Ann Intern Med 124:379–388
Fazekas F (1989) Magnetic resonance signal abnormalities in asymptomatic individuals: their incidence and functional correlates. Eur Neurol 29:164–168
Fazekas F, Niederkorn K, Schmidt R et al (1988) White matter signal abnormalities in normal individuals: correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke 19:1285–1288
Silverstone T, McPherson H, Li Q, Doyle T (2003) Deep white matter hyperintensities in patients with bipolar depression, unipolar depression and age-matched control subjects. Bipolar Disord 5:53–57
Schmidt R, Fazekas F, Offenbacher H et al (1993) Neuropsychologic correlates of MRI white matter hyperintensities: a study of 150 normal volunteers. Neurology 43:2490–2494
Schmidt R, Fazekas F, Offenbacher H et al (1991) Magnetic resonance imaging white matter lesions and cognitive impairment in hypertensive individuals. Arch Neurol 48:417–420
Moore PB, Shepherd DJ, Eccleston D et al (2001) Cerebral white matter lesions in bipolar affective disorder: relationship to outcome. Br J Psychiatry 178:172–176
Fazekas F, Kleinert R, Offenbacher H et al (1993) Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 43:1683–1689
Thomas AJ, O’Brien JT, Barber R, McMeekin W, Perry R (2003) A neuropathological study of periventricular white matter hyperintensities in major depression. J Affect Disord 76:49–54
Jorm AF, Anstey KJ, Christensen H et al (2005) MRI hyperintensities and depressive symptoms in a community sample of individuals 60–64 years old. Am J Psychiatry 162:699–705
Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ (2005) Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology 237:251–257
Novak V, Last D, Alsop DC et al (2006) Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care 29:1529–1534
Yetkin FZ, Fischer ME, Papke RA, Haughton VM (1993) Focal hyperintensities in cerebral white matter on MR images of asymptomatic volunteers: correlation with social and medical histories. AJR Am J Roentgenol 161:855–858
Acknowledgements
This research was supported by NIDDK R01060754 (A. M. Jacobson), the Herbert Graetz Fund, and in part by the Diabetes and Endocrinology Research Core NIH P30 DK36836, and in part by grant RR 01032 to the Beth Israel Deaconess Medical Center General Clinical Research Center. We thank C. Sparks and A. Burwood for their invaluable assistance in participant recruitment and study organisation.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weinger, K., Jacobson, A.M., Musen, G. et al. The effects of type 1 diabetes on cerebral white matter. Diabetologia 51, 417–425 (2008). https://doi.org/10.1007/s00125-007-0904-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-007-0904-9