Abstract
Key message
Multi-environment multi-QTL mixed models were used in a GWAS context to identify QTL for disease resistance. The use of mega-environments aided the interpretation of environment-specific and general QTL.
Abstract
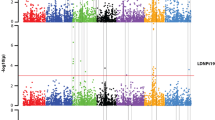
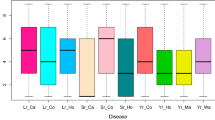
Diseases represent a major constraint for barley (Hordeum vulgare L.) production in Latin America. Spot blotch (caused by Cochliobolus sativus), stripe rust (caused by Puccinia striiformis f.sp. hordei) and leaf rust (caused by Puccinia hordei) are three of the most important diseases that affect the crop in the region. Since fungicide application is not an economically or environmentally sound solution, the development of durably resistant varieties is a priority for breeding programs. Therefore, new resistance sources are needed. The objective of this work was to detect genomic regions associated with field level plant resistance to spot blotch, stripe rust, and leaf rust in Latin American germplasm. Disease severities measured in multi-environment trials across the Americas and 1,096 SNPs in a population of 360 genotypes were used to identify genomic regions associated with disease resistance. Optimized experimental design and spatial modeling were used in each trial to estimate genotypic means. Genome-Wide Association Mapping (GWAS) in each environment was used to detect Quantitative Trait Loci (QTL). All significant environment-specific QTL were subsequently included in a multi-environment-multi-QTL (MEMQ) model. Geographical origin and inflorescence type were the main determinants of population structure. Spot blotch severity was low to intermediate while leaf and stripe rust severity was high in all environments. Mega-environments were defined by locations for spot blotch and leaf rust. Significant marker-trait associations for spot blotch (9 QTL), leaf (6 QTL) and stripe rust (7 QTL) and both global and environment-specific QTL were detected that will be useful for future breeding efforts.



Similar content being viewed by others
Abbreviations
- AMMI:
-
Additive main effect and multiplicative interaction model
- BOPA:
-
Barley oligonucleotide pool assays
- CAN_LAN:
-
Lacombe Research Center (Alberta, Canada)
- ECU_PIC:
-
“Santa Catalina” Experimental Research Station in Pichincha of the National Agricultural Research Center (INIAP, Ecuador)
- ECU_TOL:
-
“Granja Tolilla” Experimental Research Station of the National Agricultural Research Center (INIAP, Ecuador)
- GxE:
-
Genotype-by-environment Interaction
- GS:
-
Genomic selection
- GWAS:
-
Genome-wide association mapping
- ME:
-
Mega-environment
- MEMQ:
-
Multi-environment multi-QTL model
- MEX_TOL:
-
“Toluca” experimental research station of the International Center for Maize and Wheat Improvement (CIMMYT-Toluca, Mexico)
- PER_AND:
-
“Andenes” experimental research station of the National Center for Innovation in Agriculture (INIA-Andenes, Cusco, Peru)
- PER_COM:
-
“Combapata” experimental research station of the National Center for Innovation in Agriculture (INIA-Combapata, Cusco, Peru)
- QEI:
-
QTL-by-environment interaction
- QTL:
-
Quantitative trait loci
- URU_LE:
-
“La Estanzuela” experimental research station of the National Agricultural Research Institute (INIA-EELE, Colonia, Uruguay)
- URU_MC:
-
“Dr. Mario A. Cassinoni” experimental station of the Universidad de la República (UDELAR-EEMAC, Paysandu, Uruguay)
References
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57(1):289–300
Caldwell RM (1968) Breeding for general and/or specific plant disease resistance. In: Finley KW, Shepherd KW (eds) Proc. 3rd Int. Wheat Genetics Symp Aust Acad Sci. Canberra, Australia, pp 263–272
Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I (2001) Controlling the false discovery rate in behavior genetics research. Behav Brain Res 125:279–284
Bernardo R (2008) Molecular markers and selection for complex traits in plants: learning from the last 20 years. Crop Sci 48(5):1649–1664. doi:10.2135/cropsci2008.03.0131
Bernardo R (2010) Breeding for quantitative traits in plants, 2nd edn. Stemma Press (400 pages)
Bilgic H, Steffenson BJ, Hayes PM (2005) Comprehensive genetic analyses reveal differential expression of spot blotch resistance in four populations of barley. Theor Appl Genet 111:1238–1250
Bilgic H, Steffenson BJ, Hayes PM (2006) Molecular mapping of loci conferring resistance to different pathotypes of the spot blotch pathogen in barley. Phytopathology 96:699–708
Boer M, Wright D, Feng L, Podlich D, Luo L, Cooper M, Van Eeuwijk F (2007) A mixed model QTL analysis for multiple environment trial data using environmental covariables for QTLxE with an example in maize. Genetics 177:1801–1813
Bovill J, Lehmensick A, Sutherland MW, Platz G, Usher T, Franckowiak J, Mace E (2010) Mapping spot blotch resistance genes in four barley populations. Mol Breed 26:652–666
Bradbury P, Parker T, Hamblin MT, Jannink JL (2011) Assessment of power and false discovery rate in genome-wide association studies using the BarleyCAP germplasm. Crop Sci 51:52–55
Braun HJ, Rajaram S, van Ginkel M (1996) CIMMYT’s approach to breeding for wide adaptation. Euphytica 92(1–2):175–183
Caldwell RM (1968) Breeding for general and/or specific plant disease resistance. In: Finlay W, Shepherd KW (eds) 3rd International Wheat Genetics Symposium Australian. Academy of Science. Canberra, Australia, pp 263–272
Cappa EP, El-Kassaby YA, Garcia MN, Acuña C, Borralho NM, Grattapaglia D, Poltri SNM (2013) Impacts of population structure and analytical models in genome-wide association studies of complex traits in forest trees: a case study in eucalyptus GLOBULUS. PLoS One 8(11):e81267
Castro A, Chen X, Corey A, Filichkina T, Hayes PM, Mundt C, Richardson K, Sandoval-Islas S, Vivar H (2003) Pyramiding quantitative trait locus (QTL) alleles determining resistance to barley stripe rust: effects on adult plant resistance. Crop Sci 43:2234–2239
Castro A, Gamba F, Germán S, González S, Hayes PM, Pereyra S, Pérez C (2012) QTL analysis of spot blotch and leaf rust resistance in the BCD47 x Baronesse barley mapping population. Plant Breed 131:258–266
Close TJ, Bhat PR, Lonardi S, Wu Y, Rostoks N, Ramsay L, Druka A, Stein N, Svensson JT, Wanamaker S, Bozdag S, Roose ML, Moscou MJ, Chao S, Varshney R, Szucs P, Sato K, Hayes PM, Matthews DE, Kleinhofs A, Muehlbauer GJ, DeYoung J, Marshall DF, Madishetty K, Fenton RD, Condamine P, Graner A, Waugh R (2009) Development and implementation of high-throughput SNP genotyping in barley. BMC Genom 10:582
Cooper M, Messina CD, Podlich D, Totir LR, Baumgarten A, Hausmann NJ, Graham G (2014) Predicting the future of plant breeding: complementing empirical evaluation with genetic prediction. Crop Pasture Sci 65(4):311–336
Eckermann PJ, Verbyla AP, Cullis BR, Thompson R (2001) The analysis of quantitative traits in wheat mapping populations. Aust J Agric Res 52:1195–1206
Fan JB, Oliphant A, Shen R, Kermani BG, Garcia F, Gunderson KL et al. (2003) Highly parallel SNP genotyping. In: Cold Spring Harbor symposia on quantitative biology, vol. 68. Cold Spring Harbor Laboratory Press, pp. 69–78
Fan JB, Oliphant A, Shen R, Kermani BG, Garcia F, Gunderson KL, Chee MS (2003) Highly parallel SNP genotyping. In: Cold Spring Harbor symposia on quantitative biology, vol 68. Cold Spring Harbor Laboratory Press, pp 69–78
Fan JB, Gunderson KL, Bibikova M, Yeakley JM, Chen J, Wickham Garcia E et al (2006) Illumina Universal Bead Arrays. Methods Enzymol 410:57–73
FAOSTAT (2008). http://faostat.fao.org/site/339/default.aspx (verified 05 May 2011)
Federer WT (1961) Augmented designs with one-way elimination of heterogeneity. Biometrics 17(3):447–473
Germán S (2004) Breeding malting barley under stress conditions in south America. Colonia, Uruguay, pp 140–144
Germán S (2007) Roya de la hoja en cultivos de invierno: Epidemiologia de la enfermedad y comportamiento varietal. Jornada de cultivos de invierno, Young, Serie actividades de difusion 484
Gutierrez L, Cuesta-Marcos A, Castro AJ, von Zitzewitz J, Schmitt M, Hayes PM (2011) Association mapping of malting quality quantitative trait loci in winter barley: positive signals from small germplasm arrays. Plant Genome 4:256–272
Gutiérrez L, Nason JD, Jannink JL (2009) Diversity and mega-targets of selection from the characterization of a barley collection. Crop Sci 49(2):483–497
HarvEST. http://harvest.ucr.edu (verified 05 May 2011)
Hayes PM, Prehn D, Vivar H, Blake T, Comeau A, Henry I, Johnston M, Jones B, Steffenson B (1996) Multiple disease resistance loci and their relationship to agronomic and quality loci in a spring barley population. J Agric Genom 2. http://www.ncgr.org/jag/
Hayes P, Szücs P (2006) Disequilibrium and association in barley: thinking outside the glass. PNAS 103(49):18385–18386
Hayes PM, Prehn D, Vivar H, Blake T, Comeau A, Henry I, Johnston M, Jones B, Steffenson B (1996) Multiple disease resistance loci and their relationship to agronomic and quality loci in a spring-barley population. QTL http://probe.nalusda.gov:8000/otherdocs/jQTL/index.htm
Heffner EL, Sorrells ME, Jannink JL (2009) Genomic Selection for crop improvement. Crop Sci 49:1–12
Heffner EL, Jannink JL, Sorrells ME (2011) Genomic Selection accuracy using multifamily prediction models in a wheat breeding program. Plant Genome 4(1):65–75
Hickey LT, Lawson W, Platz GJ, Dieters M, Arief VN, German S, Fletcher S, Park RF, Singh D, Pereyra S, Frankowiak J (2011) Mapping Rph20: a gene conferring adult plant resistance to Puccinia hordei in barley. Theor Appl Genet 123:55–68
Hong M, Singh RP (1996) Contribution of adult plant resistance gene Yr18 in protecting wheat from yellow rust. Plant Dis 80:66–69
Jafary H, Szabo LJ, Niks RE (2006) Innate nonhost immunity in barley to different heterologous rust fungi is controlled by sets of resistance genes with overlapping specificities. Mol Plant Microb Interact 19:1270–1279
Jannink JL, Bink MCAM, Jansen RC (2001) Using complex plant pedigrees to map valuable genes. Trends Plant Sci 6:337–342
Johnson E, Miklas PN, Stavely JR, Martinez-Cruzado JC (1995) Coupling-and repulsion-phase RAPDs for marker-assisted selection of PI 181996 rust resistance in common bean. Theor Appl Genet 90(5):659–664
Kang HM, Zaitlen NA, Wade CM, Kirby A, Heckerman D, Daly MJ, Eskin E (2008) Efficient control of population structure in model organism association mapping. Genetics 178(3):1709–1723
Kraakman ATW, Niks NE, van den Berg PMMM, Stam P, van Eeuwijk FA (2004) Linkage disequilibrium mapping of yield and yield stability in modern spring barley cultivars. Genetics 168:435–446
Kraakman ATW, Martinez F, Mussiraliev B, van Eeuwijk FA, Niks RE (2006) Linkage disequilibrium mapping of morphological, resistance, and other agronomically relevant traits in modern spring barley cultivars. Mol Breed 17:41–58
Lagudah ES, McFadden H, Singh RP, Huerta-Espini J, Bariana HS, Spielmeyer W (2006) Molecular genetic characterization of the Lr34/Yr18 slow rusting resistance gene region in wheat. Theor Appl Genet 114:21–30
Lande R, Thompson R (1990) Efficiency of marker-assisted selection in the improvement of quantitative traits. Genetics 124(3):743–756
Laurie DA, Pratchett N, Snape JW, Bezant JH (1995) RFLP mapping of five major genes and eight quantitative trait loci controlling flowering time in a winter × spring barley (Hordeum vulgare L.) cross. Genome 38(3):575–585
Li J, Ji L (2005) Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity 95:221–227
Liu F, Gupta S, Zhang X, Jones M, Loughman R, Lance R, Li C (2011) PCR markers for selection of adult plant leaf rust resistance in barley (Horduem vulgare L.). Mol Breeding 28:657–666
Locatelli A, Cuesta-Marcos A, Gutiérrez L, Hayes PM, Smith KP, Castro AJ (2013) Genome-wide association mapping of agronomic traits in relevant barley germplasm in Uruguay. Mol Breed 31(3):631–654
Lorenz AJ, Chao S, Asoro FG, Heffner EL, Hayashi T, Iwata H, Smith KP, Sorrells ME, Jannink JL (2011) Genomic selection in plants: knowledge and prospects. pp 77–124 In: Sparks DL (ed) 2011. Advances in Agronomy, vol 110. Elsevier. San Diego, USA
Malosetti M, Voltas J, Romagosa I, Ullrich SE, van Eeuwijk FA (2004) Mixed models including environmental covariables for studying QTL by environment interaction. Euphytica 137:139–145
Malosetti M, Ribaut J-M, Vargas M, Crossa J, Boer M, van Eeuwijk F (2007) Multi-trait multi-environment QTL modelling for drought stress adaptation in maize. In: Spiertz JHJ, Struik PC, Van Laar HH (eds) Scale and complexity in plant systems research, gene: plant crop relations. Springer, Dordrecht, pp 25–36
Mather DE, Tinker NA, Laberge DE, Edney M, Jones BL, Rossnagel BG, Legge WG, Briggs KG, Irvine RB, Falk DE, Kasha KJ (1997) Regions of the genome that affect grain and malt quality in a North American two row barley cross. Crop Sci 37:544–554
Mathews KL, Malosetti M, Chapman S, McIntyre L, Reynolds M, Shorter R, van Eeuwijk F (2008) Multi-environment QTL mixed models for drought stress adaptation in wheat. Theor Appl Genet 117(7):1077–1091
McIntosh RA (1992) Pre-emptive breeding to control wheat rusts. Euphytica 63:103–113
Meuwissen THE, Hayes BJ, Goddard ME (2001) Prediction of total genetic value using genome-wide dense marker maps. Genetics 157:1819–1829
Meuwissen TH, Karlsen A, Lien S, Olsaker I, Goddard ME (2002) Fine mapping of a quantitative trait locus for twinning rate using combined linkage and linkage disequilibrium mapping. Genetics 161:373–379
Mott R, Flint J (2002) Simultaneous detection and fi ne mapping of quantitative trait loci in mice using heterogeneous stocks. Genetics 160:1609–1618
Muñoz-Amatraín M, Moscou ME, Bhat PR, Svensson JT, Bartoš J, Suchánková P, Šimková H, Endo TR, Fenton RD, Lonardi S, Castillo AM, Chao S, Cistué L, Cuesta-Marcos A, Forrest KL, Hayden MJ, Hayes PM, Horsley RD, Makoto K, Moody D, Sato K, Vallés MP, Wulff BBH, Muehlbauer GJ, Doležel J, Close TJ (2011) An improved consensus linkage map of barley based on flow-sorted chromosomes and single nucleotide polymorphism markers. The Plant Genome 4:238–249
Muñoz-Amatriaín M, Cuesta-Marcos A, Endelman JB, Comadran J, Bonman JM, Bockelman HE, Muehlbauer GJ (2014) The USDA barley core collection: genetic diversity, population structure, and potential for genome-wide association studies. PLoS ONE 9(4):e94688
Niks RE, Rubiales D (2002) Potentially durable resistance mechanisms in plants to specialised fungal pathogens. Euphytica 124(2):201–216
Nutter EW, Pederson VD, Foster AE (1985) Effect of inoculations with Cochliobolus sativus at species growth stages on grain yield and quality of malting barley. Crop Sci 25:933–938
Palomeque L, Li-Jun L, Li W, Hedges B, Cober ER, Rajcan I (2009) QTL in mega-environments: II. Agronomic trait QTL co-localized with seed yield QTL detected in a population derived from a cross of high-yielding adapted × high-yielding exotic soybean lines. Theor Appl Genet 119(3):429–436
Parisseaux B, Bernardo R (2004) Insilico mapping of quantitative trait loci in maize. Theor Appl Genet 109:508–514
Parlevliet JE, Zadoks JC (1977) The integrated concept of disease resistance: a new view including horizontal and vertical resistance in plants. Euphytica 26(1):5–21
Patterson HD, Williams ER (1976) A new class of resolvable incomplete block designs. Biometrika 63:83–92
Patterson N, Price AL, Reich D (2006) Population structure and eigenanalysis. PLoS Genet 2(12):e190
Pereyra S (1996) Manejo de enfermedades en cereales de invierno y pasturas, Montevideo, INIA. Serie Tecnica no 74, pp 110–111
Peterson RF, Campbell AB, Hannah AE (1948) A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can J Res 26(5):496–500
Piepho H-P (2000) A mixed-model approach to mapping quantitative trait loci in barley on the basis of multiple environment data. Genetics 156:2043–2050
Pretorius ZA, Pienaar L, Prins R (2007) Greenhouse and Weld assessment of adult plant resistance in wheat to Puccinia striiformis f.sp. tritici. Aust Plant Path 36:552–559
Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D (2006) Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38:904–909
Qi X, Niks RE, Stam P, Lindhout P (1998) Identification of QTL for partial resistance to leaf rust (Puccinia hordei) in barley. Theor Appl Genet 96:1205–1215
R Development Core Team (2005) A language and environment for statistical computing, reference index version 2.2.1. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. (verified 05 May 2011). http://www.R-project.org
Rossi C, Cuesta-Marcos A, Vales I, Gomez-Pando L, Orjeda G, Wise R, Sato K, Hori K, Capettini F, Vivar H, Chen X, Hayes PM (2006) Mapping multiple disease resistance genes using a barley mapping population evaluated in Peru, Mexico, and the USA. Mol Breed 18:355–366
Roy JK, Smith KP, Muehlbauer GJ, Chao S, Close TJ, Steffenson BJ (2010) Association mapping of spot blotch resistance in wild barley. Mol Breed 26:243–256
Saari EE, Prescott JM (1975) A scale for appraising the foliar intensity of wheat diseases. Plant Dis Reptr 59:377–380
SAS Institute (2004) Base SAS 9.1 procedures guide, vol 1–4. SAS Inst., Cary, NC
Schweder T, Spjøtvoll E (1982) Plots of p-values to evaluate many tests simultaneously. Biometrika 69(3):493–502
Singh S, Bowden R (2011) Molecular Mapping of adult-plant race-specific leaf rust resistance gene Lr12 in bread wheat. Mol Breed 28:137–142
Smith A, Cullis B, Thompson R (2001) Analyzing variety by environment data using multiplicative mixed models and adjustments for spatial field trend. Biometrics 57:1138–1147
Stavely JR (1985) The modified Cobb scale for estimating bean rust intensity. Annual report of the bean improvement cooperative (USA)
Steffenson BJ, Hayes PM, Kleinhofs A (1996) Genetics of seedling and adult plant resistance to net blotch (Pyrenophora teres f. teres) and spot blotch (Cochliobolus sativus) in barley. Theor Appl Genet 92:552–558
Stich B, Möhring J, Piepho H-P, Heckenberger M, Buckler ES, Melchinger AE (2008) Comparison of mixed-model approaches for association mapping. Genetics 178(3):1745–1754
Stracke S, Haseneyer G, Veyrieras J-B, Geiger HH, Sauer S, Graner A, Piepho H-P (2009) Association mapping reveals gene action and interactions in the determination of flowering time in barley. Theor Appl Genet 118:259–273
Szűcs P, Blake VC, Bhat PR, Close TJ, Cuesta-Marcos A, Muehlbauer GJ, Ramsay LV, Waugh R, Hayes PM (2009) An integrated resource for barley linkage map and malting quality QTL alignment. Plant Genome 2:134–140
Thomas WTB, Powell W, Waugh R, Chalmers KJ, Barua UM, Jack P, Lea V, Forster BP, Swanston JS, Ellis RP, Hanson PR, Lance RCM (1995) Detection of quantitative trait loci for agronomic, yield, grain and disease characters in spring barley (Hordeum vulgare L.). Theor Appl Genet 91:1037–1047
Tiwari KR, Penner GA, Warkentin TD (1998) Identification of coupling and repulsion phase RAPD markers for powdery mildew resistance gene er-1 in pea. Genome 41(3):440–444
Toojinda T, Baird E, Broers L, Chen XM, Hayes PM, Kleinhofs A, Korte J, Kudrna D, Leung H, Line RF, Powell W, Vivar H (2000) Mapping quantitative and qualitative disease resistance genes in a doubled haploid population of barley. Theor Appl Genet 101:580–589
Vales MI, Schon CC, Capettini F, Chen XM, Corey AE, Mather DE, Mundt CC, Richardson KL, Sandoval-Islas JS, Utz HF, Hayes PM (2005) Effect of population size on the estimation of QTL: a test using resistance to barley stripe rust. Theor Appl Genet 111:1260–1270
van Eeuwijk FA, Malosetti M, Yin X, Struik PC, Stam P (2005) Statistical models for genotype by environment data: from conventional ANOVA models to eco-physiological QTL models. Aust J Agric Res 56:883–894
Varshney RK, Marcel TC, Ramsay L, Russell J, Röder MS, Stein N, Graner A (2007) A high density barley microsatellite consensus map with 775 SSR loci. Theor Appl Genet 114(6):1091–1103
Verbyla AP, Eckermann PJ, Thompson R, Cullis BR (2003) The analysis of quantitative trait loci in multi-environment trials using a multiplicative mixed model. Aust J Agric Res 54:1395–1408
Von Korff M, Wang H, Leon J, Pillen K (2005) AB-QTL analysis in spring barley. I. Detection of resistance genes against powdery mildew, leaf rust and scald introgressed from wild barley. Theor Appl Genet 111(3):583–590
von Zitzewitz J, Cuesta-Marcos A, Condon F, Castro AJ, Chao S, Corey A, Filichkin T, Fisk SP, Gutierrez L, Haggard K, Karsai I, Muehlbauer GJ, Smith KP, Veisz O, Hayes PM (2011) The genetics of winterhardiness in barley: perspectives from genome-wide association mapping. Plant Genome 4(1):76–91
Waugh R, Jannink J-L, Muehlbauer GJ, Ramsay L (2009) The emergence of whole genome association scans in barley. Curr Opin Plant Biol 12:218–222
Yu J, Pressoir G, Briggs WH, Bi IV, Yamasake M, Doebley JF, McMullen MS, Gaut BS, Nielsen DM, Holland JB, Kresovich S, Buckler ES (2006a) A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat Genet 38:203–208
Yu J, Pressoir G, Briggs WH, Bi IV, Yamasake M, Doebley JF, McMullen MS, Gaut BS, Nielsen DM, Holland JB, Kresovich S, Buckler ES (2006b) A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat Genet 38:203–208
Yun SJ, Gyenis L, Bossolini E, Hayes PM, Matus I, Smith KP, Steffenson BJ, Tuberosa R, Muehlbauer GJ (2006) Validation of quantitative trait loci for multiple disease resistance in barley using advanced backcross lines developed with a wild barley. Crop Sci 46:1179–1186
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421
Zhu C, Gore M, Buckler ES, Yu J (2008) Status and prospects of association mapping in plants. Plant Genome 1:5–20
Acknowledgments
Funding for this project was provided by FONTAGRO (Project FTG 0617-06) and Competitive Grants program for scientific visits of the Comisión Sectorial de Investigación Científica (CSIC), Universidad de la República (UDELAR), Uruguay. The authors wish to express their appreciation for the effort of the technical personnel of all the involved institutions.
Conflict of interest
All authors have no conflict of interest. All the experiments conducted under this study comply with the current laws of all the countries in which they were conducted.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Xiaoquan Qi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gutiérrez, L., Germán, S., Pereyra, S. et al. Multi-environment multi-QTL association mapping identifies disease resistance QTL in barley germplasm from Latin America. Theor Appl Genet 128, 501–516 (2015). https://doi.org/10.1007/s00122-014-2448-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-014-2448-y