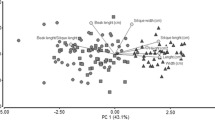
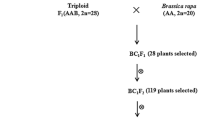
Abstract.
The frequency of gene flow from Brassica napus L. (canola) to four wild relatives, Brassica rapa L., Raphanus raphanistrum L., Sinapis arvensis L. and Erucastrum gallicum (Willd.) O.E. Schulz, was assessed in greenhouse and/or field experiments, and actual rates measured in commercial fields in Canada. Various marker systems were used to detect hybrid individuals: herbicide resistance traits (HR), green fluorescent protein marker (GFP), species-specific amplified fragment length polymorphisms (AFLPs) and ploidy level. Hybridization between B. rapa and B. napus occurred in two field experiments (frequency approximately 7%) and in wild populations in commercial fields (approximately 13.6%). The higher frequency in commercial fields was most likely due to greater distance between B. rapa plants. All F1 hybrids were morphologically similar to B. rapa, had B. napus- and B. rapa-specific AFLP markers and were triploid (AAC, 2n = 29 chromosomes). They had reduced pollen viability (about 55%) and segregated for both self-incompatible and self-compatible individuals (the latter being a B. napus trait). In contrast, gene flow between R. raphanistrum and B. napus was very rare. A single R. raphanistrum × B. napus F1 hybrid was detected in 32,821 seedlings from the HR B. napus field experiment. The hybrid was morphologically similar to R. raphanistrum except for the presence of valves, a B. napus trait, in the distorted seed pods. It had a genomic structure consistent with the fusion of an unreduced gamete of R. raphanistrum and a reduced gamete of B. napus (RrRrAC, 2n = 37), both B. napus- and R. raphanistrum-specific AFLP markers, and had <1% pollen viability. No hybrids were detected in the greenhouse experiments (1,534 seedlings), the GFP field experiment (4,059 seedlings) or in commercial fields in Québec and Alberta (22,114 seedlings). No S. arvensis or E. gallicum × B. napus hybrids were detected (42,828 and 21,841 seedlings, respectively) from commercial fields in Saskatchewan. These findings suggest that the probability of gene flow from transgenic B. napus to R. raphanistrum, S. arvensis or E. gallicum is very low (<2–5 × 10–5). However, transgenes can disperse in the environment via wild B. rapa in eastern Canada and possibly via commercial B. rapa volunteers in western Canada.
Similar content being viewed by others
References
Baranger A, Chèvre AM, Eber F, Renard M (1995) Effect of oilseed rape genotype on the spontaneous hybridization rate with a weedy species: an assessment of transgene dispersal. Theor Appl Genet 91:956–963
Beckie HJ, Hall LM, Warwick SI (2001) Impact of herbicide-resistant crops as weeds in Canada. Proc Brighton Crop Prot Conf – Weeds. British Crop Protection Council, Farnham, Surrey, UK, pp 135–142
Bing DJ, Downey RK, Rakow GFW (1996) Hybridizations among Brassica napus, B. rapa and B. juncea and their two weedy relatives B. nigra and Sinapis arvensis under open-pollination conditions in the field. Plant Breed 115:470–473
Chèvre AM, Eber F, Baranger A, Kerlan MC, Barret P, Festoc G, Vallee P, Renard M (1996) Interspecific gene flow as a component of risk assessment for transgenic Brassicas. Acta Hort 407:169–179
Chèvre AM, Eber F, Baranger A, Renard M (1997) Gene flow from transgenic crops. Nature 389:924
Chèvre AM, Eber F, Baranger A, Hureau G, Barret P, Picault H, Renard M (1998) Characterization of backcross generations obtained under field conditions from oilseed rape–wild radish F1 interspecific hybrids: an assessment of transgene dispersal. Theor Appl Genet 97:90–98
Chèvre AM, Eber F, Darmency H, Fleury A, Picault H, Letanneur JC, Renard M (2000) Assessment of interspecific hybridization between transgenic oilseed rape and wild radish under agronomic conditions. Theor Appl Genet 100:1233–1239
Darmency H (2000) Unpredictability of transgene flow between oilseed rape and wild relatives. XIeme Colloque International sur la Biologie des mauvaises herbes Dijon, France Sept, 2000
Darmency H, Lefol E, Fleury A (1998) Spontaneous hybridizations between oilseed rape and wild radish. Mol Ecol 7:1467–1473
Dolezel J (1991) Flow cytometric analysis of nuclear content in higher plants. Phytochem Anal 2:143–154
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Eber F, Chèvre AM, Baranger A, Vallée P, Tanguy X, Renard M (1994) Spontaneous hybridization between a male-sterile oilseed rape and two weeds. Theor Appl Genet 88:362–368
Eber F, LeTanneur JC, Chèvre AM (1997) Chromosome number of oilseed rape (Brassica napus L.) – wild radish (Raphanus raphanistrum) spontaneous hybrids and of their progeny estimated by flow cytometry. Eucarpia Cruciferae Newslett 19:17–18
Ellstrand NC, Prentice HC, Hancock JF (1999) Gene flow and introgression from domesticated plants into their wild relatives. Annu Rev Ecol Syst 30:539–563
Gueritaine G, Bazot S, Darmency H (2000) L'hybridization interspecifique entre le colza transgenique (Brassica napus) et la ravenelle (Raphanus raphanistrum) varie selon les individus. XIeme Colloque International sur la Biologie des mauvaises herbes Dijon, France Sept, 2000
Halfhill MD, Richards HA, Mabon SA, Stewart CN Jr (2001) Expression of GFP and Bt transgenes in Brassica napus and hybridization and introgression with Brassica rapa. Theor Appl Genet 103:362–368
Halfhill MD, Millwood RJ, Raymer PL, Stewart CN Jr (2002) Bt-transgenic oilseed rape hybridization with its weedy relative, Brassica rapa. Environ Biosafety Res 1:19–28
Hansen LB, Siegismund HR, Jørgenson RB (2001) Introgression between oilseed rape (Brassica napus L.) and its weedy relative B. rapa L. in a natural population. Genet Resour Crop Evol 48:621–627
Heap I (2002) International survey of herbicide-resistant weeds. Online. Internet. April 23, 2002. Electronic publ, available at: http://www.weedscience.org/in.asp
Heyn FW (1977) Analysis of unreduced gametes in the Brassiceae by crosses between species and ploidy levels. Z Pflanzenzüchtg 78:13–30
James C (2001) Global review of commercialized transgenic crops: 2001. ISAAA Briefs No. 24-2001 ISAAA (International Service for the Acquisition of Agri-Biotech Applications), Ithaca, New York
Jørgensen RB, Andersen B (1994) Spontaneous hybridization between oilseed rape (Brassica napus) and weedy B. campestris (Brassicaceae): a risk of growing genetically modified oilseed rape. Am J Bot 81:1620–1626
Kumar A, Rakow G, Downey RK (1998) Isogenic analysis of glufosinate-ammonium tolerant and susceptible summer rape lines. Can J Plant Sci 78:401–408
Landbo L, Andersen B, Jørgensen RB (1996) Natural hybridisation between oilseed rape and a wild relative: hybrids among seeds from weedy B. campestris. Hereditas 125:89–91
Lefol E, Danielou V, Darmency H (1996) Predicting hybridization between transgenic oilseed rape and wild mustard. Field Crops Res 45:153–161
Lefol E, Séguin-Swartz G, Downey RK (1997) Sexual hybridisation in crosses of cultivated Brassica species with the crucifers Erucastrum gallicum and Raphanus raphanistrum: potential for gene introgression. Euphytica 95:127–139
Légère A, Simard MJ, Thomas AG, Pageau D, Lajeunesse J, Warwick SI, Derksen DA (2001) Presence and persistence of volunteer canola in Canadian cropping systems. Proc Brighton Crop Prot Conf – Weeds. British Crop Protection Council, Farnham, Surrey, UK, pp 143–148
Metz PLJ, Jacobsen E, Stiekema WJ (1997) Aspects of the biosafety of transgenic oilseed rape (Brassica napus L.). Acta Bot Neerl 46:51–67
Moyes CL, Lilley JM, Casais CA, Cole SG, Haeger PD, Dale PJ (2002) Barriers to gene flow from oilseed rape (Brassica napus) into populations of Sinapis arvensis. Mol Ecol 11:103–112
Rieger MA, Potter TD, Preston C, Powles SB (2001) Hybridization between Brassica napus L. and Raphanus raphanistrum L. under agronomic field conditions. Theor Appl Genet 103:555–560
Rieger MA, Preston C, Powles SB (1999) Risks of gene flow from transgenic herbicide-tolerant canola (Brassica napus) to weedy relatives in southern Australian cropping systems. Aust J Agric Res 50:115–128
Sharma AK, Sharma A (1965) Chromosome techniques. Theory and Practice. Butterworths, London
Simard MJ, Légère A, Pageau D, Lajeunnesse J, Warwick S (2002) The frequency and persistence of canola (Brassica napus) volunteers in Québec cropping systems. Weed Technol 16:433–439
Snow AA (2002) Transgenic crops – why gene flow matters. Nature Biotechnol 20:542
Snow AA, Andersen B, Jørgensen RB (1999) Costs of transgenic herbicide resistance introgressed from Brassica napus into weedy Brassica rapa. Mol Ecol 8:605–615
Thalmann C, Guadagnuolo R, Felber F (2001) Search for spontaneous hybridization between oilseed rape (Brassica napus L.) and wild radish (Raphanus raphanistrum L.) in agricultural zones and evaluation of the genetic diversity of the wild species. Bot Helvetica 111:107–119
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Warwick SI, Beckie H, Small E (1999) Transgenic crops: new weed problems for Canada? Phytoprotection 80:71–84
Warwick SI, Francis A, La Fleche J (2000) Guide to wild germplasm of Brassica and allied crops (tribe Brassiceae, Brassicaceae) 2nd edn. Agriculture and Agri-Food Canada Research Branch Publication, ECORC Ottawa, Canada. Contribution No. 991475. [http://www.brassica.info]
Acknowledgements.
This study was supported in part by the Canadian Biotechnology Strategy fund, Government of Canada (B. rapa and R. raphanistrum), Matching Investment Initiative of Agriculture and Agri-Food Canada (AAFC), Monsanto Inc. (E. gallicum and S. arvensis), Westco Ltd. (R. raphanistrum Alberta), and the Agriculture and Agri-Food Canada research grant in support of AAFC-ECORC and INRA collaboration (R. raphanistrum). We thank Denise Maurice, Westco, Alberta; Jerry Ivany, AAFC-Charlottetown, PEI and Anne-Marie Chèvre, INRA, France, for supplying seed of R. raphanistrum from Alberta, Prince Edward Island and France, respectively; Kevin Falk, AAFC-Saskatoon Research Centre for supplying seed of B. rapa cv AC Parkland; and Monsanto Inc. for supplying seed of glyphosate-resistant B. napus. We thank the following AAFC personnel for their excellent technical assistance: Caroline Boudreault, Stephanie Ethier, Wayne Gratton, Nancy Hébert, Don Hodgins, Caroline Laberge-Pelletier, Tania Lévesque, Christopher Lozinki, Tracey McDonald, Christa Metcalfe, Connie Sauder, Constantin Voloaca and Carma Wooff.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Dvorak
Rights and permissions
About this article
Cite this article
Warwick, S.I., Simard, MJ., Légère, A. et al. Hybridization between transgenic Brassica napus L. and its wild relatives: Brassica rapa L., Raphanus raphanistrum L., Sinapis arvensis L., and Erucastrum gallicum (Willd.) O.E. Schulz. Theor Appl Genet 107, 528–539 (2003). https://doi.org/10.1007/s00122-003-1278-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-003-1278-0