Abstract
The bipedal stance and gait of theropod dinosaurs evolved gradually along the lineage leading to birds and at some point(s), flight evolved. How and when did these changes occur? We review the evidence from neontology and palaeontology, including pectoral and pelvic limb functional morphology, fossil footprints/trackways and biomechanical models and simulations. We emphasise that many false dichotomies or categories have been applied to theropod form and function, and sometimes, these impede research progress. For example, dichotomisation of locomotor function into ‘non-avian’ and ‘avian’ modes is only a conceptual crutch; the evidence supports a continuous transition. Simplification of pelvic limb function into cursorial/non-cursorial morphologies or flexed/columnar poses has outlived its utility. For the pectoral limbs, even the classic predatory strike vs. flight wing-stroke distinction and separation of theropods into non-flying and flying—or terrestrial and arboreal—categories may be missing important subtleties. Distinguishing locomotor function between taxa, even with quantitative approaches, will always be fraught with ambiguity, making it difficult to find real differences if that ambiguity is properly acknowledged. There must be an ‘interpretive asymptote’ for reconstructing dinosaur limb function that available methods and evidence cannot overcome. We may be close to that limit, but how far can it be stretched with improved methods and evidence, if at all? The way forward is a combination of techniques that emphasises integration of neontological and palaeontological evidence and quantitative assessment of limb function cautiously applied with validated techniques and sensitivity analysis of unknown variables.
Similar content being viewed by others
Introduction
Nineteenth-century scientists were quickly struck by the similarities between the pectoral (fore) and especially pelvic (hind) limbs of theropod dinosaurs such as Compsognathus and Megalosaurus on one hand and living birds on the other (Gegenbaur 1864; Huxley 1868). This similarity to them, as it still does us today, implied similar limb function and even stance, gait or locomotor dynamics. It also indicated either a remarkably detailed convergence due to the constraints of bipedalism or an ancestor-descendant relationship, the latter being the modern consensus (Gauthier 1986; Chatterjee 1997; Sereno 1999; Xu et al. 2000, 2003, 2007; Prum 2002; Zhou 2004; Mayr et al. 2005; Chiappe 2007; Senter 2007). But how similar would the terrestrial locomotion of, for example, the deinonychosaur Velociraptor and an emu (Dromaius) of comparable size be? Or how differently would the first bird Archaeopteryx and a magpie (Pica) fly? These are interesting questions of how form and function are linked (or decoupled) during evolution and how one can interpret locomotion from fossil remains. These are also less well understood or even explored research avenues, but a recent burgeoning of inquiries into locomotor and limb function in theropods (including extant birds) prompts us to review progress in this field. We will show how the study of theropod locomotor function and evolution has evolved and has great scientific potential as long as its limitations are kept in mind.
In this review, we focus on studies of theropod locomotor function (including perspectives from functional morphology and biomechanics) by covering terrestrial and aerial locomotion and then related aspects of limb function (e.g. prehension, climbing, swimming). General aspects of dinosaur and more basal archosaur locomotor function or general biomechanics were reviewed elsewhere recently (Christiansen 2000; Padian 2001; Paul 2002; Zhou 2004; Alexander 2006; Hutchinson and Gatesy 2006; Hutchinson 2006; Chiappe 2007). In particular, Farlow et al. (2000) and Gatesy (2002) gave thorough reviews of theropod locomotion, so we centre our treatment on theropod locomotion studies since ∼2000. To exemplify the value of and high methodological standards for empirical studies of locomotion in extant theropods, we have integrated some of the more significant recent studies of extant bird locomotion into our review as a step towards improved synthesis of neontological and palaeontological perspectives in evolutionary biomechanics and morphology.
Discussion
Why study theropod locomotion?
First, why do researchers bother reconstructing theropod locomotion? We see at least four reasons. As the introduction intimates, a major reason is to understand how the bipedal stance and gait of birds evolved—which traits are truly novel for crown group birds (here Neornithes; also often termed Aves)? Or, how far back can we trace more ancestral traits down the theropod family tree, and which are the oldest traits? Flight and bipedal locomotion both are important adaptations of birds (and other theropods), but how were their component novelties assembled and modified, or how did aerial/terrestrial locomotor performance (turning or running ability, takeoff or jumping capacity, etc) change over time? Such questions are fundamental pursuits in evolutionary biology, including natural history and the study of adaptations, novelties, ‘evolvability’ and constraints. Furthermore, systematic and morphological studies have revealed many anatomical characters that might have functional importance (e.g. modifications of bone shapes and proportions or muscle/tendon scars). Studies that carefully tie such anatomical forms to locomotor function help to reveal the biological importance of such characters and may generate novel hypotheses of relatedness, as well as bringing such static, esoteric osteology ‘alive’ in the eyes of non-specialists.
In turn, a second reason is that locomotor function in specific taxa can be of interest, particularly when linked with a broader question. For example, the running and turning abilities of large tyrannosaurs have been debated (Paul 1988, 1998, 2008; Farlow et al. 2000; Hutchinson and Garcia 2002; Hutchinson 2004a, b; Sellers and Manning 2007)—not just because tyrannosaurs were interesting animals in their own right (and dismissing their celebrity status) but also because the issue is pertinent to broader questions about how very large size influences locomotion in land animals (Alexander 1989; Biewener 1989, 1990)—how would a 6+ tonne biped stand and move since no such phenomena can be observed today? It would be comparatively uninformative to merely reconstruct how a theropod taxon within the body size range of extant taxa (e.g. the medium-sized Early Jurassic Dilophosaurus) moved, as taken in isolation such animals are of limited use to studies of the evolution of locomotion in theropods or locomotion in general.
A third reason for examining theropod locomotion is because so many broader, complex questions will ultimately hinge upon how individual theropods moved and how the motion of individual clades evolved. In particular, palaeoecological and broader macroevolutionary considerations such as predation, competition, migration/biogeography and the interpretation of diversification/extinctions patterns as adaptive radiations and co-evolution depend to varying degrees upon accurate reconstructions of theropod locomotion as one of the principal kinds of evidence.
A fourth reason is more a methodological one, but one that palaeontology has struggled with since its inception as a discipline: What are the limits of resolution for reconstructing past life? Given how much is unknown about extinct animals and how poorly known some aspects of locomotor function in extant animals are, should we just avoid asking how they moved? We see this as a rather anti-scientific viewpoint, but still one we encounter frequently. Successful reconstruction of locomotion in any extinct taxon, if it remains explicit and rigourous about the assumptions and unknowns involved in that reconstruction, should be considered a triumphant demonstration of the value, diversity and rigour of palaeobiological inquiry and honest, careful science. Yet, to date, such successes are relatively simple and tentative (Hutchinson and Garcia 2002; Hutchinson and Gatesy 2006; Sellers and Manning 2007; Gatesy et al. 2008)—surely we can do better, but how much better? What are the limits of knowledge and how close to them are we? Functional reconstructions have obvious value as entertainment, but are less appreciated as valuable tools in science education. They can demonstrate how science can confront daunting ambiguities yet still make wary progress without crossing the hazy line into fantasy.
Terrestrial locomotion
Overview of locomotor evolution
Extant birds are bipedal and fairly cursorial (proportionately long-legged; reviewed further below) because their theropod ancestors were at least facultative bipeds (and probably obligate bipeds quite early in their history) and had fairly cursorial limbs. The ancestral mode of locomotion for archosaurs (and saurians in general) is reconstructed as quadrupedal and ‘hip driven’, which entails rotation of the entire pelvic limb with appreciable power input from large, extrinsic tail-based musculature (Gatesy 1990, 1995, 1999a, b; Carrano 1998, 2000; Hutchinson and Gatesy 2000). Striding bipedalism in extant birds is often characterised as ‘knee driven’—progression during walking is achieved by rotation of the elongate lower limb by flexors and extensors of the strongly flexed knee. In walking birds, muscles that ancestrally assisted with rotation of the whole limb by flexion and extension of the hip (hip-driven locomotion) instead act to stabilise the short, robust and subhorizontally positioned femur (Gatesy 1990, 1999a; Carrano 1998; Hutchinson and Gatesy 2000). This dichotomy between hip and knee-driven locomotion breaks down somewhat when birds run, however; femur retraction by hip extensor muscles increases, but still is relatively small compared with the rotation arcs in more basal saurians (Gatesy 1990, 1995, 1999a, b). At such faster speeds, birds gradually transition, with only subtle kinematic changes, into a ‘grounded run’ (Rubenson et al. 2003) involving a bouncing gait without an aerial phase; only at substantially faster speeds do some species have a true aerial phase in running (e.g. Gatesy 1999a).
To some degree, the hip-driven mechanism seems linked to a more caudally positioned centre of mass of the body, whereas a more cranially positioned centre of mass may be correlated with a more knee-driven mechanism. The evolutionary transition between these mechanisms is illuminated by tail reduction and pectoral limb expansion (thus a presumed centre of mass shift) from basal theropods to birds (Gatesy 1990, 1995; Figs. 1, 2). Footprints and anatomical and biomechanical evidence support the inference that some fast running capacity was plesiomorphically present in theropods and inherited by modern birds, but there was much homoplasy in this athletic capacity, particularly related to body size changes (reviewed below). Whilst the mechanics and control of theropod bipedalism might seem to be concentrated in the parasagittal plane, the latest evidence (also reviewed below) shows that this is a major oversimplification; substantial three-dimensional (3D) dynamics are (and always were) involved (Hutchinson and Gatesy 2000; Rubenson et al. 2007).
Phylogeny of theropod dinosaurs. Compiled from Gauthier (1986), Sereno (1999), Clarke et al. (2006), Senter (2007), Xu et al. (2007) and Clarke and Middleton (2008). Only major clades and taxa mentioned in the text are shown. Illustrations along the right side correspond to taxa in the clades immediately to their left and are (top to bottom): Coelophysis (illustration by Frederik Spindler), Baryonyx, Allosaurus, Tyrannosaurus, Compsognathus, Chirostenotes, Velociraptor (latter six illustrations by Scott Hartman), Microraptor (illustration by Jim Robins), Archaeopteryx, Confuciusornis (latter two illustrations by Scott Hartman), Longipteryx, Patagopteryx, Ichthyornis (latter four images from Chiappe 2007), Struthio (ostrich) and Gallus (chicken). Images are used with permission or copyright free. Body size changes are only roughly shown; images are not to scale
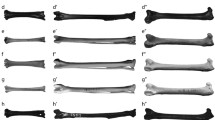
a Evolution of theropod pelvic limbs. Right pelvic limbs of (from left to right; data sources and Fig. 1 clade names in parentheses): Dilophosaurus (Theropoda/Coelophysoidea; from unpublished data); Allosaurus (Tetanurae/Carnosauria; from unpublished data), Velociraptor (Eumaniraptora/Deinonychosauria; from Hutchinson et al. 2008) and extant emu Dromaius (Neornithes/Palaeognathae; from Goetz et al. 2008). Arbitrary mid-stance walking poses are shown to exemplify one of almost infinite conceivable steps of gradual transformations from more to less upright poses across theropod evolution, but emu pose is empirical data from Goetz et al. (2008). The right femur is shown in proximal (above) and caudal (below) views to the right of each limb. Ancestral accessory femoral/acetabular articulations are shown there in purple (iliofemoral) and scarlet (ischiofemoral); unified neornithine antitrochanter articulation in violet. Yellow arrow (ITC label) indicates line of action for M. iliotrochantericus caudalis (see text). Inset shows muscle moments about the knee joint (red and white circle)—muscle moment equals muscle force (Fmusc) times muscle moment arm (rmusc). Centre of mass (black and yellow circle) and ground-reaction force vector (red arrow) shown. b Hypotheses of theropod locomotor evolution from ancestral archosaur to derived avian styles. Top to bottom: evolutionary continuum, stepped transition distributed across several nodes or abrupt one-step dichotomy
At least three hypotheses are conceivable for how theropod terrestrial gaits evolved between these two mechanisms (Fig. 2b); we view the temporally diffuse trends displayed in the available evidence (reviewed by Gatesy 2002; Hutchinson 2006) as favouring a gradual transition rather than major steps concentrated at only one or a few nodes. The study of terrestrial locomotion of theropods now stands at a juncture where the question is, when and in what manner did theropods transition from ancestral patterns of limb orientation and neuromuscular control to patterns essentially identical to that inherited by crown group birds? More specifically, can we reliably determine which specific species or at least broad theropod clades had more or less derived aspects of limb positioning and control or how specific muscles and other anatomical components changed to enable this transition (e.g. Gatesy 1990; Carrano 2000; Hutchinson and Gatesy 2000)?
Neontological studies: empirical analyses of extant bird bipedalism
Whilst the reconstruction of locomotion of extinct theropods has enjoyed a renaissance since the paradigm-shifting work of Alexander (1985, 1989) and Gatesy (1990; also Gatesy et al. 1999), studies of neornithine terrestrial locomotion are in the midst of an even greater explosion of research. A comprehensive review of this literature would be a worthy effort in itself; in the previous section, we attempted a brief overview. Here, we cite these studies in the hope that researchers studying extinct dinosaur locomotor mechanics will become more conversant in it. This explosion is fueled by a recognition by comparative biomechanists and physiologists that birds are an excellent, and often lamentably overlooked, model system for bipedal locomotion (Gatesy 1990, 1999a, b; Gatesy and Biewener 1991; Reilly 2000; Abourachid 2001; Zeffer and Norberg 2003; Rubenson et al. 2003, 2007; Hancock et al. 2007; Smith et al. 2007; Usherwood et al. 2008), ontogenetic scaling (Smith et al. 2006; Main and Biewener 2007), pelvic limb muscle–tendon interactions and task-dependent functions (Gabaldon et al. 2004, 2007; Nelson et al. 2004; Roberts et al. 2007; Azizi et al. 2008; Higham et al. 2008; Higham and Biewener 2008; Higham and Nelson 2008; Nelson and Roberts 2008), neuromechanical control and stability, or unsteady locomotion (Earls 2000; Roberts and Scales 2002; Biewener and Daley 2007; Daley and Biewener 2003; Daley et al. 2006, 2007; Jindrich et al. 2007; Kurz et al. 2008), links between mechanics and energetics (Roberts et al. 1998a, b; Bundle et al. 1999; Griffin and Kram 2000; Roberts 2001; Ellerby et al. 2003, 2005; Marsh et al. 2004, 2006; Marsh and Ellerby 2006; Ellerby and Marsh 2006; McGowan et al. 2006; Rubenson et al. 2006; Ellerby and Marsh 2006) and musculoskeletal pathobiology (Abourachid 1993; Corr et al. 2003, 2007; Goetz et al. 2008).
Climbing, perching and ‘unsteady’ locomotor behaviours such as standing/sitting, jumping, hopping/skipping and turning remain almost unstudied for crown group birds in a modern biomechanical and physiological, let alone phylogenetic, context (but see Bonser 1999; Earls 2000; Henry et al. 2005; Jindrich et al. 2007), so reconstructions of these activities for extinct theropods (Hutchinson et al. 2007; Stevens et al. 2008) still have weak empirical grounding.
Functional morphology—how are theropod form and function related?
As the most obvious and accessible evidence for theropod locomotion is skeletal (and ichnological; see below), it might seem that scaling and biomechanical theory could use these data to independently test the hypotheses from above (Fig. 2). Carrano (1998, 2001) and Carrano and Biewener (1999) have conducted elegant, integrative analyses of bone loading patterns in extant taxa (especially birds), directly relating bone loading to bone geometry, and then investigated how the scaling of bone geometry illuminates locomotor patterns in extinct taxa. Their findings added empirical weight to the general inferences (above) on theropod locomotor evolution. Yet, bone biomechanical analyses have not yet covered all feasible avenues for dinosaurs (e.g. finite element analysis), whereas bone scaling studies largely have (see Hutchinson 2006 and references therein).
However, adequate reading of the osteological record requires establishment of a strong and specific link between form and function. This link is not yet forged for pelvic limb joint structure in theropods, although some important groundwork has very recently been laid (e.g. Rubenson et al. 2007; Hertel and Campbell 2007; Goetz et al. 2008). Thus, assumptions that limb orientation during standing or moving can reliably be reconstructed from joint geometry or other osteological data (e.g. Paul 1988, 1998, 2008) are premature, yet compelling, subjects for examination (e.g. Pontzer et al. 2006).
Hertel and Campbell (2007) provided useful measurements of angulations of the antitrochanter (of the hip joint) and fibular condyle (of the knee joint) in crown group birds, showing a potential link to locomotor kinematics (yet still undemonstrated by quantitative kinematic studies). Leaving aside the important issue that the roles of the soft tissues that produce and control limb movement in living theropods are still poorly understood (but see neontological references above), they made some important errors that are unfortunately still common in theropod studies.
First, Hertel and Campbell (2007) misinterpreted antitrochanter morphology and evolution in non-neornithine theropods. As Farlow et al. (2000) and others (e.g. Novas 1996) described and depicted, the antitrochanter refers to an acetabular structure that is clearly an articular surface, not a muscle attachment site like the ornithischian ‘antitrochanter’ (an unfortunate misnomer) and maniraptoran (including neornithine) processus supratrochantericus (Hutchinson 2001a). In basal theropods and related taxa, it is plesiomorphically a two-part, ‘kidney-shaped’ (Novas 1996) structure with separate iliac and ischial components. Our manipulations of a wide range of theropod fossils, summarised in Fig. 2a, suggest that the proximal femur plesiomorphically had two articulations with these surfaces (varying with limb abduction and flexion). The first, iliac part (including the supra-acetabular crest in more basal taxa) mainly articulated with the femoral head/neck (fossa articularis antitrochanterica). In some taxa and limb positions, the medial surface of the femoral head may have contacted the pubis as well. The second, ischial part mainly articulated with the caudomedial proximal femur (corresponding to the intertrochanteric fossa in more basal Reptilia). This articulation transformed in tetanuran theropods as the femoral head became more medially inflected (Carrano 2000; Hutchinson 2001b), reducing the ischial articulation and expanding the iliac antitrochanter. As a result, the antitrochanter-fossa articularis antitrochanterica articulation gradually became the predominant one. The dinosaurian antitrochanter is homologous (but secondarily modified) with the condition in crown group birds and is present in some basal non-neornithine birds (pers. obs.), but easily abraded or obscured by displacement of the pelvis as in some specimens of Archaeopteryx (Hertel and Campbell 2007: Fig. 10a). This is a prime example of the benefits of a transformational, phylogenetic approach to character evolution (Hutchinson 2001a) over a simplified, essentialistic view. We know that avian hip articulations must have evolved in some way from the plesiomorphic archosaurian condition, and theropod fossils show the way via intermediate character states (Prum 2002).
Second, as many preceding studies have done, Hertel and Campbell (2007: p.800) described bird knee and ankle joints as moving ‘restricted to the parasagittal plane.’ This longstanding assumption has recently been falsified by Rubenson et al. (2007) for ostriches. Although ostriches surely have some unique joint specialisations, the general findings probably apply to all crown group birds (see Gatesy 1999a) and possibly all theropods as well. The knee joint in particular is not hinge-like; movements at all joints have substantial off-parasagittal, 3D motions. This is partly why Hutchinson and Gatesy (2000) focussed on 3D kinematics and kinetics in their analysis of the evolution of theropod limb control.
Not only are theropod limb motions and joint geometry 3D but essentially all muscles act in three dimensions (e.g. with moment arms in flexion/extension, ab/adduction and medial/lateral long-axis rotation; see Hutchinson et al. 2005, 2008). It is almost impossible for muscles to produce moments (rotational forces; moment arms multiplied by muscle forces; Fig. 2) purely in flexion/extension, and even small non-parasagittal moments could play important roles in limb function. The idea of ‘one muscle, one function’ is a misleading oversimplification, falsified by recent analyses (Gabaldon et al. 2004; Roberts et al. 2007; Higham et al. 2008; Higham and Biewener 2008; Higham and Nelson 2008; Nelson and Roberts 2008).
For example, Rubenson et al. (2006) contended that the guinea fowl M. iliotrochantericus caudalis (Fig. 2)—the function of which has informed previous interpretations of the evolution of hindlimb support in theropods (Hutchinson and Gatesy 2000)—acts as a hip extensor instead of (or as well as) a medial rotator (as in Hutchinson and Gatesy 2000). We observe that it is anatomically capable of both in birds and generating an abduction moment as well; these 3D functions should be highly dependent on 3D joint orientation. Our inspections of homologous muscles in musculoskeletal models of related theropods (Hutchinson et al. 2005, 2008; Goetz et al. 2008) support the inference that this major muscle’s hip moment arm (especially for more caudal fibres) may switch from flexion to extension at extreme joint angles, and this is much more likely to occur than for its medial rotation capacity. Regardless, Hutchinson and Gatesy (2000) and Rubenson et al. (2006) are in agreement that this and other muscles are important for supporting the body during the stance phase of locomotion. This underscores the importance of a 3D approach to muscle function, kinematics and kinetics in studies of theropod locomotor function, a theme which we shall return to in discussing flight evolution. Whilst this considerably complicates such analyses, the recognition of this point should demonstrate the growing maturity and sophistication of scientific inquiry in this area. The field has grown beyond simple 2D kinematic studies, although where inquiry remains at an early stage, such simplifications still have merit (see below). Further progress in this area depends on more detailed 3D experimental (especially in vivo and simulation) analyses of joint morphometrics, kinematics and kinetics in crown group birds.
Whilst investigations of gross bone morphology have been very fruitful and remain promising, more microscopic approaches to theropod osteology in relation to locomotor function are underexploited (but see Pontzer et al. 2006). An unexpected but exciting development in recent years has been the gradual integration of histological and ontogenetic with scaling and biomechanical perspectives on theropod locomotion. Smith et al. (2006, 2007) provided an extensive dataset on ostrich pelvic limb muscular architecture that would be useful for phylogenetically bracketing (Witmer 1995) quantitative soft tissue anatomy in extinct theropods. Erickson et al. (2004) and Bybee et al. (2006) used histological sectioning of long bones and ontogenetic changes of limb proportions in tyrannosaurids (also see Hutchinson 2004b) and the carnosaur Allosaurus to infer that growth was rapid (∼20 years to skeletal maturity) and allometric (resulting in shorter, more robust distal bones with higher strength than in isometric scaling). This is consistent with the inference of locomotion-related ecological changes during ontogeny, such as shifts from pursuit to ambush predation (e.g. Erickson et al. 2004). Some major ecological changes are hardly unlikely considering the ∼1,000× body mass increase expected from tyrannosaurid hatchlings to adults (Erickson et al. 2004).
The simple relative proportions of theropod limb bones are often used for assessing locomotor capabilities (e.g. Christiansen 1999; Coombs 1978; Holtz 1995; Paul 1988, 1998). However, Gatesy and Middleton (1997) found that non-avian theropod limb proportions were relatively homogeneous (∼5% limb length differences), suggesting that perhaps the small differences between limb proportions in theropod taxa have been over-emphasised by previous studies. Hutchinson (2004b) showed that even subtle differences in posture (and by extrapolation, limb proportions) can have important implications for limb loading and muscle mechanics. Yet, such implications are too complex to be safely inferred from limb proportions alone; they must be demonstrated by biomechanical analysis (see below).
Gatesy and Middleton (1997, 2000; also Carrano 1998) also found that theropod limb proportions overlap with those in basal birds, with a subsequent increase of disparity within birds. To the degree that function and anatomical form are linked, then, this supports the inference that there was functional continuity from early through later non-avian theropods and thence into birds. This and a wealth of anatomical and biomechanical data (summarised above and below) falsify the dichotomy of entirely distinct ‘theropod’ and ‘avian’ modes of locomotion (Jones et al. 2000; Hertel and Campbell 2007), which is a misreading of the work of Gatesy (1990), Carrano (1998), Farlow et al. (2000) and others. Non-avian theropod and avian theropod locomotion evolved (Fig. 2), a hypothesis supported by multiple independent lines of evidence (cited previously here) and consilient with phylogenetic analyses nesting birds within Coelurosauria.
What then, if anything, should be concluded from more or less ‘cursorial’ limb proportions and anatomy (e.g. carnosaurs vs. tyrannosaurids) in specific theropods? Cursoriality is best defined as suite of morphological specialisations (e.g. long, gracile distal limb elements and hinge-like joints; Coombs 1978). Its links with higher-level functional, behavioural or performance factors are multifarious (e.g. not just running performance but also endurance, locomotor economy or efficiency, home range size, body size, and posture; Carrano 1999 and references therein) and not well understood for extant, let alone extinct, taxa. Carrano (1999) showed that theropods retained the cursorial limb design of ancestral dinosaurs, but gradually reduced it with large size and enhanced it with small size, with much homoplasy among lineages (Holtz 1995). The label cursorial is a useful descriptive term (Carrano 1999; Farlow et al. 2000) but it is a priori reasoning to relate it to specific locomotor performance in extinct theropods.
As an example of these pitfalls, Jones et al. (2000) attempted to use pelvic limb functional morphology to show that the oviraptorosaur Caudipteryx was instead a secondarily flightless bird, using a locomotor mechanism fundamentally unlike that in theropods and like that in birds (i.e. knee driven). Yet, evidence for the gradual transformation of hip into knee-driven bipedalism (above; Fig. 2), flaws in their dataset for limb proportions and their reconstructions for centre of mass calculations of Deinonychus and Caudipteryx, a lack of any sensitivity analysis and a priori assumptions biasing the results mean that this study’s conclusions can be dismissed (Christiansen and Bonde 2002; Dyke and Norell 2005). Abundant, consilient data from independent systematic studies have established the close relationship of Caudipteryx with other oviraptorosaurs and perhaps therizinosaurs (Fig. 1), contradicting the notion that any of these taxa are avian (Sereno 1999; Mayr et al. 2005; Senter 2007; Xu et al. 2007).
Another exemplar of the problems with simple dichotomies in locomotor function features studies by Paul (1988, 1998, 2008), who hypothesised that large tyrannosaurs (and other theropods) could run as fast as or faster than living rhinoceroses or horses, based on cursorial limb proportions and ‘permanently flexed’ rather than columnar limbs, among other evidence. We term this the ‘tachylocomotor megapredator’ hypothesis. As animal limb proportions span a wide continuum (Carrano 1999) and are only indirectly related to running performance (summarised above), this evidence from cursorial anatomy is ambiguous. Living and extinct animals falsify the dichotomy between ‘flexed or columnar’ limb poses (Hutchinson et al. 2005; Ren et al. 2008). No modern studies explicitly envisage large theropods as truly columnar (limb joint angles of about 180°). The controversy has implicitly been about how flexed their limbs were or in other words where their typical limb joint angles were on a continuum of poses (Gatesy et al. 2008). This field is moving on from naïve dichotomies. Biomechanical and fossil footprint (ichnology) studies are a way forward.
Biomechanical modelling/simulation of running mechanics in theropods
Biomechanical models and simulations of theropod locomotion have primarily focussed on bone ‘strength indicators’ (Alexander 1985, 1989; Christiansen 1998), maximal speed or running ability (Blanco and Mazzetta, 2001; Hutchinson and Garcia 2002; Hutchinson 2004a, b; Blanco and Jones 2005; Hutchinson et al. 2005, 2007; Sellers and Manning 2007; Gatesy et al. 2008) and turning capacity (Carrier et al. 2001; Henderson and Snively 2003; Hutchinson et al. 2007). As there is no strong indication from experimental or theoretical analyses that bone strength is an important (let alone primary) limitation on running speed, bone strength indicators (e.g. Mazzetta et al. 1998, 2004; Blanco and Jones 2005) may overestimate relative running ability, particularly if muscle strength is more limiting, so approaches that investigate more stringent limits on locomotor performance may have longer-term value.
Blanco and Mazzetta (2001) used a simple but novel and creative biomechanical model to estimate running speeds in the carnosaur Giganotosaurus, assuming that the ability to get each foot under the body with each step (time taken to retract one hindlimb and protract the next) was a crucial constraint on running speed. There were dubious assumptions in this model (e.g. centre of mass located at the hip, use of an inverted pendulum model of locomotion that is more appropriate for walking than running), little sensitivity analysis, and insufficient presentation of data purported to show that the model worked reasonably well for humans and ostriches. Hence, its result of a speed estimate of 14 ms−1 should be viewed as questionably demonstrated, with an uninvestigated, potentially wide margin of error.
Hutchinson and colleagues (Hutchinson and Garcia 2002; Hutchinson 2004a, b; Hutchinson et al. 2005, 2007; Gatesy et al. 2008) have used simple quasi-static models of bipedal running to estimate how massive the antigravity (extensor) muscles of each hindlimb would need to be to support the large vertical ground-reaction forces at mid-stance of fast running in 19 different species, extant and extinct. They did not estimate maximal speeds except by rough comparison with mid-stance forces and corresponding duty factors (for Tyrannosaurus, estimated at ∼5–11 ms−1; Hutchinson et al. 2004b), which should be tightly correlated (Weyand et al. 2000; Rubenson et al. 2003; Usherwood and Wilson 2005a, b).
The benefits of this modelling approach are its (1) relative simplicity, (2) support as a reasonable method based upon dozens of empirical studies (results and references in Hutchinson 2004a), (3) ‘validation’ from application to extant taxa combined with appropriate sensitivity analysis of unknown parameter values, (4) mechanistic link to experiments showing the importance of limb forces (and hence muscle forces) for speed and ground-reaction force capacity (Weyand et al. 2000; Usherwood and Wilson 2005a, b) and (5) inclusion of the major parameters likely to play a role in theropod limb mechanics (particularly mid-stance vertical ground-reaction forces, muscle architecture and moment arms, limb orientation (Fig. 3) and centre of mass position). It makes the assumptions, reasonably justified, that mid-stance of locomotion can be modelled as a quasi-static situation (ignoring inertia and aligning the foot’s centre of pressure with the centre of mass of the body; e.g. Biewener 1989), muscles are isometrically active (i.e. their tendons would be doing any lengthening/shortening required; Biewener and Roberts 2000; Alexander 2002; Roberts 2002), and involves static optimisation, avoiding consideration of temporal effects (e.g. from the mechanical demands imposed early or late in the stance phase of locomotion; Anderson and Pandy 2001).
Biomechanical models of the importance of limb orientation for theropod pelvic limb mechanics. Models of Tyrannosaurus (3D models from data in Hutchinson et al. 2005) are posed in poses corresponding to models ‘Trex_1’ (a), ‘upright’ (b), and ‘columnar’ (c) in Hutchinson (2004b). Differences in antigravity muscle masses (as percent of body mass) required to sustain fast running (mid-stance ground-reaction forces of 2.5 times body weight; speed over 11 ms−1) are shown next to the respective joints (from top to bottom, hip, knee, ankle and toes; numbers in parentheses represent approximate flexor, not extensor, muscle masses). These exemplify that differences of limb pose can drastically alter muscle force requirements and hence locomotor performance. Thus, knowing which pose theropods used is important, with functional effects too complex to easily predict without biomechanical analysis
Sellers and Manning (2007) provided a substantial methodological advance for theropod locomotor studies by using the model data of Hutchinson (2004a, b), combined with estimations of protractor muscle anatomy, tendon dimensions and body segment inertia in a dynamic 2D simulation that estimated maximal running speeds for a human, ostrich and emu and five extinct theropods. Their results for extant taxa compared well with approximate maximal speeds, and the running stride length–speed relationships for extinct taxa fell within the broad ranges predicted for typical extant animals, ground-truthing their method. They obtained results agreeing with those of Hutchinson (2004a, b) and colleagues (above; perhaps unsurprising as they used the same data). Estimated maximal speed for Tyrannosaurus was ∼8 ms−1 (but with a range of uncertainty from 5 to 11 ms−1; their Fig. 4) with maximal absolute speed increasing strongly with decreasing body mass, up to ∼18 ms−1 for the 3 kg coelurosaur Compsognathus. The latter result might be an artefact of their method, which does not account for biomechanical constraints on running speed that may vary between larger and smaller taxa (Gatesy et al. 2008), as well as differing relative limb muscle masses, but this awaits further testing.
One of their more novel results came from their sensitivity analysis of the assumed values for maximal muscle contraction velocity and limb muscle mass in Tyrannosaurus (Sellers and Manning 2007: Fig. 4). They discovered that both factors limit speed equally at ∼8 ms−1 running speed (contraction velocity 8 lengths/s; muscle mass 15% body mass/leg), whereas if muscle mass was larger, contraction velocity became more speed limiting. To our knowledge, this tradeoff has never been demonstrated for extant taxa, so this finding may be of broader relevance for understanding the factors limiting sprinting speed more generally in animals (Weyand et al. 2000; Usherwood and Wilson 2005a, b).
Nonetheless, like any modelling approach, this study has its assumptions and limitations, many acknowledged warily by Sellers and Manning (2007). The limb tendon slack lengths (at which tendons generate no passive force) were estimated using the same limb poses for all taxa. This did not account for postural variation due to evolutionary changes of morphology and body mass (Gatesy 1990; Biewener 1989; Gatesy and Biewener 1991). The poses at which tendons would have been slack are important unknowns (but see Gatesy et al. 2008). As slack lengths have major influences on running simulations (Scovil and Ronksy 2006), this raises cautions for simulations including tendons, important as they are for keeping muscle activity close to isometric maximal force, storing and returning elastic strain energy and smoothing motions (e.g. Alexander 2002; Roberts 2002). Likewise, as in Hutchinson’s studies (above), musculoskeletal anatomy was presumed to be proportionately identical in all taxa, whereas there is clear evidence it was not; theropod anatomy evolved (Hutchinson 2001a, b; 2002; Hutchinson et al. 2008). This is a more difficult issue to resolve.
Sellers and Manning (2007) used a simple point (at the distal metatarsus) to represent the foot and toe joints, which lacked muscles or tendons or a detailed foot–ground interaction. Thus, it is unlikely that the simulations produced plausible ground-reaction forces, an advantage that the models of Hutchinson and colleagues have over this approach. The limb poses found by the simulations vary widely (Sellers and Manning 2007: Fig. 1), and some involve large-amplitude pitching motions of the trunk. The study’s assumption that the trunk centre of mass lay cranially along the vertebral axis, rather than being displaced cranioventrally from the hips, would cause inaccurate estimates of hip joint moments (Hutchinson 2004b; Hutchinson et al. 2007) and could partly explain the wide variation of trunk poses found. Overall, compared with Hutchinson and colleagues’ earlier studies, this approach is superior for quantifying absolute speeds, but not necessarily superior for dealing with poses or ground-reaction forces (Gatesy et al. 2008). Yet generally, these studies are in firm agreement, ironically falsifying Paul’s (2008) prediction that they would be in conflict.
Considering these limitations and the wide range of unknown parameters in any simulations, the quantitative results must be viewed as rough estimates. Similar to Hutchinson and colleagues’ studies, the likely margin of error is ∼50% (Sellers and Manning 2007: Fig. 4)—quantitative precision is impossible. This ambiguity complicates efforts to distinguish locomotor performance of individual taxa or trace the evolution of locomotor performance. This field of biomechanically modelling extinct organisms is still young, and there is hope that the breadth of uncertainty will decrease with new discoveries, data (from extant or extinct taxa) and methods. Nonetheless, the study by Sellers and Manning (2007) pushes the frontiers of inquiry into how extinct theropods may have moved, as well as the fundamental biomechanics of running, into promising new ground.
Biomechanical methods incorporate the anatomical specialisations (large muscle attachments, limb proportions and pose, etc.) featured in the tachylocomotor megapredator hypothesis (above) of Paul (1988, 1998, 2008) as qualitative evidence for ‘fast’ running capacity and show that these features have less quantitative (or even opposite) impact on athleticism than expected (e.g. Hutchinson 2004b; Hutchinson et al. 2005; Gatesy et al. 2008). For example, Paul (2008) misconstrued biomechanical modelling as being unable to distinguish running specialisation in ostriches vs. humans on the basis of relative muscle masses. But, this not was what quantitative analyses argued or assumed. Indeed, a biomechanical approach shows that the more limiting factors for running ability are muscle moments (forces times moment arms or leverages, Fig. 2a). Ostriches can produce about 1.9, 1.6 and 1.5 times the hip, knee and ankle maximal isometric extensor muscle moments that humans of comparable size can. Incidentally, this is effectively the quantified version of Paul’s (1988, 1998, 2008) qualitative arguments for high speed in large theropods. These maximal joint moments are respectively about six to 15, three to 12 and two to six times what ostriches may need for fast running, depending on input assumptions about limb pose and body centre of mass (lower values probably the most accurate ones; data from Hutchinson 2004a; also limb poses from Rubenson et al. 2007). Muscle mass is only part of the biomechanical calculations (with fascicle length, determining the physiological cross-sectional area of muscle and hence its maximal force output). Larger moment arms allow greater moments to be generated by a given muscle and seem more responsible for the biomechanical differences in athleticism.
Where does this leave the controversy over running in large theropods? We admit as participants that we are tiring of the debate, which seems to be drawing to a relative consensus. Our opinion is that the question of what locomotor performance was like in any theropod is a quantitative question, which requires quantitative methods to address. As the range of error in those quantifications is broad, ultimately their conclusions resemble qualitative ones, but with the advantage that they can address vagaries in the methods and evidence that qualitative approaches cannot. We intend no disrespect, but by refusing to adopt such a quantitative approach, Paul (1988, 1998, 2008) has, in relative isolation as a dissenter, essentially left the debate.
Relative agreement has been reached (Hutchinson 2004b; Sellers and Manning 2007; Gatesy et al. 2008) that (1) model solutions exist that support the hypothesis of at least slow running in large theropods (in agreement with comparative anatomy), (2) speed estimates are 5–11 ms−1, not plausibly approaching 20 ms−1 (a quantitative question that comparative anatomy cannot sufficiently address) and (3) large theropods ran with non-columnar limbs (in agreement with joint anatomy; Paul 1988), but then so do elephants and other large animals (Ren et al. 2008)—the real issue is that the degree of joint flexion is unknown but can be quantitatively bounded within a range of possibility (Gatesy et al. 2008). If some consensus is forming, why then should researchers persist in reconstructing tyrannosaur (and other theropod) locomotion? We return to this in the conclusion.
Turning biomechanics—could a large theropod spin on a dime?
In addition to body size, body shape changed tremendously during theropod evolution, with presumably strong influences on locomotor function (Gatesy 1990). Carrier et al. (2001); Henderson and Snively 2003; Gatesy 2001a, 2003; Milan et al. 2004; Gatesy et al. 2005 showed how general body shape related to an increase in turning ability within theropods, especially birds, as rotational inertia about the dorsoventral axis decreased with shortening of the tail. On this basis, Hutchinson et al. (2007) estimated, based on very simple biomechanical calculations of maximal hip rotator muscle torques needed to overcome inertia (still requiring more thorough experimental tests), that an adult Tyrannosaurus might have taken 2–3 s to turn 45°. Jindrich et al. (2007) showed how contrastingly adept ostriches are at turning, able to turn the body 19° in a sidestep turn in 0.22 s and turning smoothly with minimal changes of joint kinematics or kinetics. Bipedal humans, in contrast, have a turning design more than two times as effective (due to their vertical body posture) as ostriches’. This study’s empirical advances could improve estimates of dinosaur turning performance and evolution.
Carrier et al. (2001) went so far as to conclude that non-avian theropods ran with strongly elevated tails and torsos (a ‘jackknifed posture’) to optimise turning performance. Yet, this conclusion does not hold well against the bulk of anatomical evidence from tail vertebrae and hip joint articular surfaces (Paul 2005), which indicates a more subhorizontal orientation of the body and tail with generally gentle spinal curvature. Paul’s (2005) argument that overshortened ischium-based hip extensor muscles provide additional evidence against the reconstruction of Carrier et al. (2001) is less convincing as the quantitative anatomy of those muscles is uncertain and the capacity of other hip extensors (ilium and tail-based) to compensate was not addressed, nor were the quantitative biomechanical forces, moments or work required of the muscles involved. Tail flexibility and its effects on turning potential are as yet unexamined from a biomechanical perspective and are a critical aspect in which human and ostrich/bird comparisons fail for most non-avian theropods.
Turning ability or ‘agility’ (however, one defines this biomechanically ambiguous term) should not only be reflected by general body form but also by form–function relationships within the appendages. The foot–substrate interface is where the most marked specialisations might be expected. Snively and Russell (2002) presented the first finite element analysis of dinosaurian limb mechanics (which have lagged behind the number of studies of cranial mechanics) as part of a broader analysis of theropod metatarsal mechanics that involved phylogenetic, morphometric, morphological and physical and computational modelling analyses (Snively and Russell 2003; Snively et al. 2004). Like a later finite element study of ornithopod dinosaur feet (Moreno et al. 2007), the assumed kinetics and kinematics were quite simple, to date untested by application to extant taxa (or comparison with experimental bone strain data; e.g. Main and Biewener 2007) and full of uncertainties. Yet, the results qualitatively supported a role for the unusual mediolaterally constricted ‘arctometatarsalian’ (Holtz 1995) structure in sharing loads among metatarsals II–IV and providing a stiff (but not immobile) functional unit better able to sustain high or off-axis loads. The functional importance of the arctometatarsalian pes is thus clarified, but the evolutionary factors underlying its independent evolution (four times in coelurosaurs; Snively et al. 2004) remain open to speculation.
Footprints—how did extinct theropods position their feet?
Important finds of new fossil trackways (sequences of fossil footprints) in recent years have expanded our knowledge of theropod locomotion, such as clearly didactyl tracks of deinonychosaurian theropods (Fig. 4a; the footprint ichnogenera Dromaeopodus and Velociraptorichnus; Kim et al. 2008; Li et al. 2008). As most other theropod footprints are not easily diagnosable to particular subclades, this latter discovery in particular might hold exciting promise for uncovering details about deinonychosaur locomotion (and thus the evolution of theropod locomotion), for example, how intermediate their pelvic limb function was. However, a plausible method for testing such an inference does not yet exist.
Significant recent theropod fossil footprint discoveries. a Didactyl deinonychosaur trackway Dromaeopodus (modified from Li et al. 2008; circles indicate two footprints); b Zygodactyl avian trackway Shandongornispes (Lockley et al. 2007; image provided by Martin Lockley); c unusual ?Triassic–Jurassic? possible avian footprints Gruipeda (Melchor et al. 2002; image provided by Ricardo Melchor). All images are used with permission and have been scaled to the same length; scale bars are 1 m in a and 1 cm per unit shown in b, c
Ichnology has a growing importance for revealing the diversification of avian locomotor form and function, such as the zygodactyl avian tracks discovered by Lockley et al. (2007; Fig. 4b). Perhaps most remarkably, Melchor et al (2002; de Valais and Melchor 2008; Genise et al. 2008) reported on puzzling ‘bird-like’ Late Triassic/Early Jurassic footprints from Argentina, placed in the footprint ichnogenera Gruipeda (Fig. 4c) and Alaripeda. These are characterised by high interdigital angles, small size and gracile proportions, a reversed hallux imprint even in shallow tracks and other features commonly thought to be exclusive to birds (e.g. Farlow et al. 2000). They are of much interest because they could indicate a relatively earlier origin of birds than most palaeontologists suspect, although this depends strongly on stratigraphic correlation and dating, which remains open to interpretation (Chiappe 2007; de Valais and Melchor 2008). The authors were astutely circumspect about their identification (Melchor et al. 2002, p. 937: ‘an unknown group of theropods showing avian characters’) as it is not clear at which node of the theropod phylogeny these features optimise as synapomorphic for (i.e. whether these features are most accurately called eumaniraptoran, avian, ornithurine, etc.; see Carrano and Wilson 2001) or how much convergent evolution of foot(print) form there might be among theropod lineages. The footprints’ indication of an at least partly retroverted hallux, however, strongly suggests a position deeper within Aves (see discussion of hallucal retroversion further below). Regardless, the footprints are important for reconstructing the evolutionary of form and function in Mesozoic theropods.
The field of dinosaur ichnology has been rapidly maturing in recent years, moving beyond naïve classification of ichnospecies with qualitative, two-dimensional simplifications, into rigourously detailed and even quantitative description, physical sectioning or 3D imaging (e.g. Farlow et al. 2000; Gatesy 2001a, 2003; Milan et al. 2004; Gatesy et al. 2005; Manning 2004, 2008; Bates et al. 2008; Platt and Hasiotis 2008) as well as experimental (Milan 2006; Milan and Bromley 2006, 2008) and computational analysis (Gatesy et al. 1999; Henderson 2003, 2006). Although much like for osteological and other fossil remains, the fundamental discovery, description and analysis of new theropod trackways will always be a vital driving force for this field; it is currently an exciting time for methodological developments and conceptual enhancements. For example, promise of totally new insights is offered by relating patterns of skin impressions in footprints to stance phase foot kinematics and substrate kinetics (Gatesy 2001a), potentially allowing shallow tracks, not just deep ones (Gatesy et al. 1999; Gatesy 2003) to reveal how theropod feet moved. However, as Gatesy (2001a) laments, a persistent challenge is that foot mechanics and substrate interactions in extant birds remain very poorly understood. Thus, ichnologists who push that frontier forward will likely contribute the most to reconstructing dinosaur locomotion from the substrate up.
Many studies have rather unquestioningly followed Alexander (1976) and subsequent analyses (e.g. Thulborn, 1990) in estimating dinosaur speeds from preserved footprint lengths and stride lengths. Despite some faults (Hutchinson and Gatesy 2006; Gatesy et al. 2008), Henderson’s (2003) computer modelling approach shows that the conventional approach of assuming that hip height (required for estimating speed) is equal to four times footprint length is as good as or better than any other approaches. However, just how good is ‘good’? As Alexander (1991) cautioned, trackway speed estimates (e.g. if compared with known speeds) are frequently off by factors of 200% or more. Errors and ambiguities in estimations of hip height (Henderson 2003; Gatesy et al. 2008) and foot length (Henderson 2006; Milan and Loope 2007; Manning 2008) for extinct taxa likewise impact speed estimates. Hence, no trackway speed estimate can be assumed to be very accurate (Alexander 1991), even though trackways are somewhat direct evidence of behaviour.
Similarly, the use of trackways to distinguish between walking and running gaits is more difficult than sometimes presumed (discussed for an example below). Thulborn’s (1990) classification of reconstructed relative stride lengths (trackway apparent stride length divided by estimated hip height or leg length) into walking (<2.0), trotting (<2.9) and running (>2.9) gaits suffers from several major limitations that compel us to recommend very cautious application. Hip heights and leg lengths of trackmakers are always rough estimates (Alexander 1991; Henderson 2003; Gatesy et al. 2008), and thus, relative stride lengths are not empirically well-grounded data, compounded by the preservational inaccuracies for stride length estimates from trackways (Manning 2004, 2008; Henderson 2006). Moreover, extant animals often do not fit within the classification boundaries used by Thulborn (1990) and others. In particular, there is no trotting (or a discrete ‘jogging’) gait in bipeds (trotting is one of several footfall patterns used by quadrupeds), so this term is inappropriate for theropod locomotion and should be abandoned. However, at relative stride lengths >2.9, most birds and other species are running (Gatesy and Biewener 1991; Gatesy 1999a; Rubenson et al. 2003; Hancock et al. 2007), albeit not necessarily with an aerial phase, so trackways beyond this rough boundary (but not necessarily in the ‘trotting’ range of 2.0–2.9) might indicate the use of bouncing (biomechanically running) gaits. We recommend that comparisons of slower bipedal trackways (relative stride lengths <2.9) at most be limited to quantitative comparisons of estimated relative stride lengths and not make qualitative inferences about gaits from such data.
Day et al. (2002; also Mossman et al. 2003) described exciting new Middle Jurassic mid-sized theropod trackways (sadly now landfill-obscured) in which the same animal accelerated during a few strides across ∼60 m, adopting a more narrow positioning of the feet. It is not clear that the animal was running (see previous paragraph; also further above on speed estimate errors), as the estimated relative stride lengths are only moderately long (∼2.9). Nonetheless, the trackways are valuable in showing how one individual, at one moment in time, was changing its speed. Much has been made of ‘wide-gauge’ footprints in theropods but in our view, while the patterns of foot placement are interesting and important data, they need not indicate radical changes of locomotor mechanics or major differences among taxa. For example, the illustrated foot placements still seem sufficiently narrow that the feet would still have been positioned medial to the hip joints, requiring the actions of the same muscles to prevent over-adduction of the limbs (Hutchinson and Gatesy 2000), just perhaps slightly more active in wider-gage trackways. Unfortunately, interpretation of such tracks is sorely impeded by a dearth of quantitative empirical data on how mediolateral foot placement changes with speed in extant birds (one exception is for penguins; Kurz et al. 2008) or how it relates to broader patterns such as muscle function or centre of mass motions (Kuo 1999).
Regardless, the Day et al. (2002) and other recently discovered trackways are exciting evidence of non-steady-state locomotion in theropods, enlarging our window onto their behaviour. This is no trivial point, for extinct theropod locomotion surely encompassed a range of activities broader and more ecologically important than walking or running at a steady speed. Further evidence of other behaviours including sitting/crouching (Milan et al. 2004), limping (Lockley et al. 1994) and swimming (see below) is also now available. Integration of such trackway data with other locomotor/behavioural evidence should be a high priority for future research, inspired by earlier syntheses such as Gatesy et al. (1999), Farlow et al. (2000) and Henderson (2003).
Aerial locomotion
Our review of aerial locomotion is organised into coverage of the origin of flight (especially wing-assisted incline running and ‘four-winged’ dinosaurs), the evolution of the wing stroke and the early evolution of flight. Again, a review of the massive amount of neontological research on the biomechanics and functional morphology of flight in crown group birds is beyond this article’s scope as it dwarfs even the literature on terrestrial locomotion. Excellent reviews are in Hedenström (2002), Videler (2006) and Tobalske (2007). Advances such as digital particle velocimetry (Spedding et al. 2003; Warrick et al. 2005; Hedenström et al. 2006), pressure transducers (Usherwood et al. 2003, 2005), as well as new insights into muscle physiology (especially sonomicrometry; e.g. Hedrick et al. 2003) and comparative analyses (e.g. Tobalske et al. 2003; Altshuler and Dudley 2002; Altshuler et al. 2004) have driven progress in this research domain at least as rapidly as for terrestrial locomotion. Middleton and Gatesy (2000; also Nudds et al. 2004; Gatesy and Middleton 2006; Dyke and Nudds 2008) provided valuable overviews of pectoral limb anatomical disparity (occupation of morphospace). Investigation of to what degree this is linked to functional disparity in extant clades might reveal clearer links with flight performance than that for cursorial limbs and running performance (see above) and would help close the largely artificial gap between palaeontological and neontological theropod studies.
Origin of flight: not just the same old stories
Virtually every conceivable story for the origin of flight in theropods can be found in the literature (see Padian 2001 and other references in the same volume; Zhou 2004; Chiappe 2007), but most have not met with much enthusiasm due to flawed methodology (see Ma et al. 2002’s critique of Videler 2000), lack of (or contradictory) evidence, over-emphasising single taxa such as Archaeopteryx rather than ancestral nodes and phylogenetic optimisation (Chatterjee and Templin 2003, 2004; and references therein; see discussion in Gatesy 2002) and other flaws (e.g. Kaiser 2000; Videler 2000, 2006; Long et al. 2002, 2003). Earls’s study (2000) is remarkable in that it is grounded in experimental data from living birds, a shortcoming of many of the aforementioned studies. Even the most prominent recent studies such as the ‘pouncing proavis’ model of Garner et al. (1999) and Burgers and Chiappe’s (1999) thrust-winged runner origin of flight have largely been eclipsed or absorbed by two recent studies: the hypothesis of Dial (2003a) and colleagues’ of a wing-assisted incline running (WAIR) origin of flight and the discovery of four-winged dinosaurs such as Microraptor both of which will be discussed in detail next.
Ontogenetic-transitional wings and the ‘parkour’ origin of flight
Just as it seemed that study of the origin of flight was entering detente and researchers were refocussing their attention onto the origin of the flight stroke (Padian 2001), Dial (2003a) dropped a bombshell. He inferred that poorly feathered wings in juvenile chukar partridges (and subsequently other species) could be used to ascend steep slopes by using aerodynamic wing-flapping forces to increase the legs’ ground-reaction forces, pushing them against the substrate and increasing traction (summarised by Dial 2003b; Hutchinson 2003; Summers 2003; Dial et al. 2006). The functional, ecological and morphological transitions during neornithine ontogeny match those expected during theropod phylogeny, providing a robust third major hypothesis for flight origins. The origin of theropod flight could have happened in almost any non-aquatic setting on Earth, a ‘wherever’ (or for those familiar with recent athletic fads, parkour) origin of flight.
Unlike many other theropod flight origin hypotheses, the WAIR hypothesis has been strengthened in light of new data from extant theropods, suggesting that it might have staying power. Bundle and Dial (2003) added more critical experimental data on the biomechanics of partridge pectoral and pelvic appendages during walking, flight and WAIR, showing that their previous predictions from kinematics had empirical support from kinetics. Furthermore, Tobalske and Dial (2007) used advanced modern flight biomechanics including digital particle image velocimetry to tease apart the relative contributions of wing inertia, profile drag and lift to the observed ‘spoiler-like’ wing function in WAIR. They demonstrated that wing lift was the dominant contributor, even in small ‘proto-winged’ chicks. Peak lift during the WAIR downstroke was 140% body weight in adults rather than the 220% observed by Bundle and Dial (2003), which presumably incorporated some wing inertia contributions. Tobalske and Dial (2007) surprisingly found in that even young chicks had wings of sufficient aerodynamic properties to produce sustained circulation. This aerodynamic circulation persisted despite the presence of symmetrical feathers in the chicks, features long presumed to be aerodynamically incompetent. Instead, the chicks had poor WAIR (and flight) performance related to underdeveloped wingbeat kinematics, producing small vortex loops with long wingbeat durations. Remarkably, the authors speculated that, in extant birds, neural control or muscle mechanics are more important to flight and WAIR than wing or feather aerodynamics.
The next generation of the WAIR hypothesis, rebranded as the more inclusive ontogenetic-transitional wing (OTW) hypothesis (Dial et al. 2008a), added several new insights: (1) As gliding and soaring are derived behaviours in crown group birds, they are irrelevant to flight origins, whereas even immature birds use flap-running ‘proto-flight’ (e.g. WAIR) to the exclusion of gliding; (2) the plesiomorphic neornithine wing stroke acts in consistent orientations across ontogeny and behaviours in the global and gravitational reference frames (Gatesy and Baier 2005), but is behaviourally flexible in the classical vertebral (i.e. anatomical) reference frame; and (3) the flexible wing stroke of birds can be used to ascend—or descend—3D terrain safely. As such, the flap-running wing stroke appears as a valid precursor to flight in non-avian theropods, considering the essential similarity of simply feathered forelimbs or ‘proto-wings’ in ontogenetic and phylogenetic transitional forms, the ability of proto-wings to produce limited aerodynamic forces and the evident utility of even such limited forces for the navigation of rough terrain. The recognition that extant birds use varying degrees of aerodynamic forces in substrate-based locomotion also provides a plausibly seamless adaptive continuum between ‘running’ and ‘flying’, with the consequence that attempts to define any one phylogenetic node as the transition between the two may be futile.
Although equally plausible as concepts, neither the classical arboreal nor cursorial hypotheses are supported by the existence of extant taxa that flap their gliding membranes for proto-flight or use running to glide (Dial et al. 2008a). The OTW hypothesis currently has the strength of coherent, phylogenetically cogent neontological evidence over its competitors, and we feel that it represents the currently best supported hypothesis regarding the origin of theropod flight.
Were there four-winged dinosaurs?
However, significant obstacles for the OTW hypothesis may exist—in particular the growing recognition that some theropods close to the origins of birds had pelvic limbs with a shocking amount of feathering, even extending down onto the metatarsi (Fig. 5). To date, the taxa Microraptor (Xu et al. 2003), Pedopenna (Xu and Zhang 2005a, b), Archaeopteryx (Christiansen and Bonde 2004; Longrich 2006), Confuciusornis (Zhang et al. 2006) and some enantiornithine birds (Zhou and Zhang 2004) have at least some contour feathering on their pelvic limbs, in cases quite substantial and even asymmetrically constructed ‘flight feathers’, leading to suggestions that these were four-winged, gliding animals (e.g. Xu et al. 2003). This condition might have been ancestral for Eumaniraptora, including birds and deinonychosaurs—more evidence on the distribution and degrees of pelvic limb feathering in taxa around this node is sorely needed. Some small, conceivably gliding/flying forms such as Rahonavis still have unresolved phylogenetic positions (e.g. within Deinonychosauria or Aves), and even a single specimen, such as Epidexipteryx (Zhang et al. 2008), which has unfeathered legs, could change character optimisation of when/how many times ‘feathered trousers’ or some degree of flight capacity evolved (e.g. Chiappe 2007).
Putative ‘four-winged’ dinosaurs Microraptor gui (a holotype fossil from Xu et al. 2003; b left lateral view ‘adducted limb’ reconstruction by Jim Robins; d dorsal view abducted limb reconstruction from Xu et al. 2003; e dorsal view ‘biplane’ reconstruction from Chatterjee and Templin 2007) and Archaeopteryx lithographica (c; dorsal view ‘abducted limb’ reconstruction from Longrich 2006). All images are used with permission and are scaled to same snout-tail tip length in a, b and c–e
A key problem in interpretating pelvic limb plumage lies in the inadequate testing of whether these feathered pelvic limbs formed viable flight surfaces in multiple taxa around the node Eumaniraptora, which is quite controversial (Padian 2003; Padian and Dial 2005; Zhang et al. 2006; Chatterjee and Templin 2007). Microscopic (including perhaps histological or high resolution non-invasive imaging) analysis of attachment of these putative flight feathers is required before their potential for aerodynamic function can be considered more than speculative. This would also help evaluate Chatterjee and Templin’s (2007) rather a priori reconstruction, asserting on aerodynamic grounds that the metatarsal feathers in Microraptor must have been dislocated during fossilization. Until then, their ‘biplane’ reconstruction, the published hypothesis with the best support from aerodynamic analysis (Longrich’s (2006) study is similar but more basic), is only one of several possibilities. Likewise, conjectures that these feathers would cause too many difficulties for running locomotion or would provide streamlining (Longrich 2006; Chatterjee and Templin 2007) deserve proper testing. Given their position and extent, it might be surprising if at least some of these leg feathers did not have some degree of aerodynamic integrity (Xu et al. 2005; Zhang et al. 2006)— much as ontogenetic-transitional proto-wings do (Dial et al. 2008a). Nonetheless, the palaeobiological mantra ‘exceptional claims require exceptional evidence’ certainly applies here, as the reconstructed anatomy and flight modes in four-winged dinosaurs are so unlike those in extant theropods that a robust foundation of multifaceted evidence is mandated. The capacity of eumaniraptorans to abduct their hindlimbs (Longrich 2006) deserves close, cautious examination, as this could constrain aerodynamic performance. As the feathered trousers of extant raptors have been adduced as analogues for this behaviour (Chatterjee and Templin 2007), neontological studies of their aerodynamics should prove insightful. Whilst the OTW hypothesis is well-supported by neontological evidence, it may ultimately require re-assessment in view of this revolutionary palaeontological evidence. It is yet to be seen how the two will be reconciled. However, both lines of evidence support the inference that there were degrees of intermediacy between flight and substrate-based locomotion (be that terrestrial or arboreal), weakening the concept of a simple dichotomy between ground-bound ancestors and aerially adept avian descendants (see Paul 2002 for an alternative scenario).
Where did the wing’s flight stroke come from?
Considering the WAIR/OTW hypothesis and mounting evidence that at least some coelurosaurian theropods could have occupied arboreal or other three-dimensional habitats (see ‘Climbing and arboreality’ below), recent research has eliminated the venerable ‘ground up or trees down’ dichotomy. Padian (2001) noted that it is nigh impossible to be more specific than this, urging a refocus onto the question of the evolutionary origin of the wings’ flight stroke. This challenge has been taken up by some subsequent studies.
Gatesy and Baier (2005) codified how the kinematics of pectoral limb motions should be compared among non-flying and flying taxa in order to reconstruct flight stroke evolution, revealing that previous analyses had not fully addressed the homologies of motions intrinsic to the flight stroke and its precursor motions. They found that the ‘predatory strike’ motions reconstructed in other studies (e.g. Gishlick 2001; Padian 2001) generally involved motions opposite to the downward path of the wing’s flight stroke when expressed in an anatomical (e.g. glenoid fossa) coordinate space. Rather than conclude that the predatory strike was non-homologous with the flight stroke, they cautioned that kinematics had been over-emphasised. Critical missing ingredients in reconstructing the evolution of the flight stroke include muscular, bony, inertial, gravitational and aerodynamic or substrate reaction forces. For example, the aerodynamic forces in a small, non-volant coelurosaur with even lightly feathered pectoral limbs could have been substantial (e.g. Dial et al. 2008a). They urged a stronger focus on the sequence of evolutionary steps in whole-limb mechanics preceding the origin of flight, not just the immediate predecessor to the flight stroke, and outlined four criteria (glenoid reference frame, kinetic consistency, kinematic homoplasy and behavioural continuity) for reconstructing these steps. Hence, perhaps the simple dichotomy between a predatory strike and a flight-competent wing stroke is insufficient.
Far from merely providing a methodological framework, the authors proceeded to deliver the goods. They (Baier et al. 2007) used simple biomechanical modelling to reconstruct the transition from an active muscular force-balance shoulder mechanism in basal archosaurs to a passive one in basal birds (at least Ornithothoraces) that was supported by an acrocoracohumeral ligament (Fig. 6). This also solved the mystery of what the ‘biceps tubercle’ (coracoid tuberosity) and other structures on fossil theropod pectoral bones related to: These were ligament attachment sites that were intermediate steps in the evolution of the derived ligament-balancing system. Baier et al. (2007) have thereby illuminated a novel, overlooked transformational sequence for parts of the wing-stroke mechanism. In coming years, it will be exciting to see other transitional pieces identified, further reconstructing the assembly and modification of the flight mechanism.
Continuum of shoulder force-balance mechanisms in Theropoda across the origin of flight (modified from Baier et al. 2007; used with permission). Right scapula-coracoids in lateral view of representative taxa from left to right (Fig. 1 clade names in parentheses): Alligator, Sinornithoides (Deinonychosauria: Troodontidae), Sinornithosaurus (Deinonychosauria), Archaeopteryx (Aves), Confuciusornis (Aves) and Columba (extant pigeon; Neognathae). Dotted lines represent the approximate line of action of the acrocoracohumeral ligament (reorienting across the phylogeny); shaded lines represent the approximate areas of origin of shoulder protractor muscles (reducing across the phylogeny)
What happened after the origin of flight?
The flight apparatus evolved substantially within birds with the expansion of the pectoral skeleton (Gatesy 2002; Paul 2002; Zhou 2004; Gatesy and Middleton 2006; Chiappe 2007) and its associated musculature (Jasinoski et al. 2006; Baier et al. 2007) and feathers, as well as changes of the tail (Gatesy 1990, 2001b), central nervous system control (already specialised in Archaeopteryx; Alonso et al. 2004) and the ventilatory apparatus that fueled aerobic flight (Codd et al. 2008). The basal ornithurines/ornithuromorphs Yanornis, Yixianornis and Hongshanornis (Fig. 1; Zhou 2004; Zhou and Zhang 2003) exhibit a well-developed flight apparatus (generally quite comparable with that of basal neornithines; Clarke and Middleton 2008) and may be some of the earliest wading-specialised birds. Along with other taxa, they support the inference that pectoral limb flight function, as in the pelvic limbs, had approximated the ancestral neornithine condition in Ornithurae.
The role of the tail in the evolution of flight has at times seen undue neglect in favour of the pectoral apparatus, but lately interest has grown. The oviraptorosaur Nomingia (Barsbold et al. 2000) exhibits a parallel evolution of ‘pygostyle’ (fused distal tail vertebrae) morphology that is part of a suite of tail specialisations within this theropod side branch (Sereno 1999; Senter 2007; Xu et al. 2007) that seems to relate little to the evolution of birds or flight. A study of Zhongornis (Gao et al. 2008) showed that reduction of caudal number preceded formation of the pygostyle. The ornithurine Yixianornis and its relatives (Clarke et al. 2006) were the first taxa to have a ‘ploughshare-shaped pygostyle’ correlated with the presence of retricial bulb and tail fanning (Baumel 1988; Gatesy and Dial 1996). This study concluded that the rod-like tail present in more basal, non-ornithurine birds was just stiffened and reduced, with only two retrices attached until this pygostyle morphology evolved. The evolution of retricial feathering itself was covered by Gatesy and Dial (1996), Gatesy (2001b), and Zheng et al. (2007). Again, the clade Ornithurae (Fig. 1) seems to be where the caudal appendage of theropods achieved its major functional aspects inherited by crown group birds.
Thus, it is relatively uncontroversial that flight evolved within birds prior to the massive diversification in the crown group, but there has been little but qualitative functional morphology studies done to date. An exception is the biomechanical estimation of wing loading in basal birds (Sans et al. 2002), which estimated that wing loading decreased by ∼50% from feathered non-avian theropods to basal birds, and reviewed evidence for the origin of the alula (‘bastard wing’) in Ornithothoraces. Perhaps there is now sufficient palaeontological and neontological evidence on flight form and function to proceed further with quantitative biomechanical studies of flight evolution.
Other aspects of limb function in theropods
Here, we cover grasping functions of the limbs, climbing, perching and arboreality and finally aquatic locomotion in theropod dinosaurs.
Predatory grasping motions as a predecessor to the flight stroke?
As the flight stroke is so critical for understanding flight evolution, what non-locomotor behaviour preceded it? Gauthier and Padian’s (1985) classic reconstruction of putative pectoral limb motions in non-volant maniraptoran theropods (building on JH Ostrom’s earlier work) supported the feasibility of the avian flight stroke as being exapted from ancestral predatory motions (as above). With this study and the seminal work of Jenkins (1993), presumably in mind, in the last few years, there has been a burgeoning of similar studies on the mobility of the pectoral limb skeletons of theropods. Gishlick (2001) presented a more detailed revision of the Gauthier and Padian (1985) analysis, whereas Chatterjee (1997; also Chatterjee and Templin 2004) presented a less compelling reconstruction for climbing pectoral limb motions presaging the flight stroke.
Senter and colleagues in particular have quantified the maximal potential ranges of joint motion in numerous taxa throughout theropod phylogeny, including the bizarre ceratosaur Carnotaurus (Senter and Parrish 2006), the large carnosaur Acrocanthosaurus (Senter and Robins 2005), the important Late Jurassic basal maniraptoran Ornitholestes (Senter 2006a), the oviraptorosaur Chirostenotes (Senter and Parrish 2005), the enigmatic and controversial alvarezsaurid Mononykus (Senter 2005) and the dromaeosaurids Deinonychus and Bambiraptor (Senter 2006b), as well as a survey of avian scapular orientation (Senter 2006c). Carpenter (2002; also Lipkin and Carpenter 2008) provided a general survey of forelimb mechanics and motions in theropods (Coelophysis, cf. Coelurus, Allosaurus, Deinonychus and Tyrannosaurus).
These studies show that within coelurosaurian theropods, compared with more basal taxa, the manual digits generally became less flexible (with some exceptions; Senter and Parrish 2005), while the more proximal joints (elbow and shoulder) seem to have increased their ranges of motion for elevation and protraction (Senter and Parrish 2005; Senter and Robins 2005; Senter 2006a). This increase of proximal joint flexibility coincides with relative elongation of the proximal limb segments involved (e.g. Chatterjee and Templin 2004; Bybee et al. 2006). In turn, these less constrained pectoral limb joint motions were exapted for flight in birds, with later clades evolving further flight-related modifications such as proper wing folding (Carpenter 2002; but see Gishlick 2001; Paul 2002). Whilst these studies provide useful data on what motions and poses were osteologically impossible and show how theropod forelimb motion constraints evolved (including specialisations along side branches), they still leave very wide latitude for how theropods actually moved their limbs, as soft tissue biomechanical data which should greatly constrain ranges of motion much further (Sellers and Manning 2007; Gatesy et al. 2008) have not yet been well integrated into these approaches (Gatesy and Baier 2005).
Interaction of pectoral limb predatory function with prey surface topology, size or vulnerabilities remains almost unexplored. However, Manning et al. (2008) used simple biomechanical modelling and physical robotic testing to infer that dromaeosaurid pedal claws (the hypertrophied digit II ungual in particular) were climbing tools, rather than slashing/disemboweling weapons, in agreement with Carpenter (2002). There are many uncertainties involved, particularly the importance of the pedal velocity (modelled as 2 and 11 ms−1) and force orientation (modelled as similar to vertical forces in high-speed running), as in other biological tissues, the skin of likely prey should have had material properties that were highly dependent upon loading rate and direction. As dromaeosaurid biting was presumed to be the primary method of dispatching prey, studies of jaw relative to claw mechanics could be quite informative. Regardless, this study provided a novel perspective and clever integration of biomechanical methods that should inspire further studies.
Climbing and arboreality—which theropods might have spent time in trees?
Given the availability of trees and other non-horizontal substrates throughout the Mesozoic, the idea of climbing or arboreal theropods seems reasonable and has been previously proposed (Paul 1988, 2002; Naish 2000; Witmer 2002). However, recent evidence has made the strongest case yet—as previously predicted, non-avian feathered theropods of quite small size, bearing curved claws, have been discovered (Xu et al. 2000, 2003; Zhang et al. 2002). So far, none seem to have been highly specialised for arboreality, but then, this is questionable even for some Mesozoic birds (Glen and Bennett 2007). Climbing proficiency or dynamics have not been quantitatively estimated for any non-avian theropods. In particular, the role of the tail in climbing is still a speculation far from general acceptance (Chatterjee 1997; Zhou 2004).
Middleton (2001) provided a critical perspective on how a reversed hallux (‘perching toe’ a caudally directed first digit of the pes) can be defined and identified in extant birds, which holds much promise for settling which extinct birds had one. He emphasised that hallucal retroversion spans a continuum from unreversed through partly reversed to highly retroverted. A caudal position of the fossa for metatarsal I and a longitudinal twisting of the metatarsal I shaft are the two best indices of hallucal retroversion—preserved position is not reliable as it is too subject to taphonomic distortion. In contradiction to some previous studies, hallucal orientation in non-avian theropods is uniformly unreversed, a condition preserved relatively unaltered in basal birds (e.g. Rahonavis, Archaeopteryx; Mayr et al. 2005, 2007) until some point within Ornithothoraces. Yet, this hardly precludes access to arboreal environments in any theropods; size (of climber and substrate) was probably the main constraint on climbing capacity, as in extant animals.
Glen and Bennett (2007) revisited an old conundrum of theropod claw (ungual) design: Can one predict what substrates individual taxa might have frequented (from highly terrestrial to highly arboreal) based upon claw geometry alone? In stark contradiction to some earlier studies (Feduccia 1993; Yalden 1997; but see Peters and Gorgner 1992; Chiappe 1997; Hopson 2001; Pike and Maitland 2004), they found that basal birds had claw shapes more typical of ground foragers than predominantly arboreal forms and overlapped with non-avian theropods. This is in broad agreement with the thorough study by Hopson (2001) using manual and pedal proportions of phalanges, but conflicts with evidence from manual phalangeal proportions consistent with some degree of arboreality in some basal birds (Zhou and Farlow 2001).
Could most extinct theropods swim?
Few if any studies have seriously doubted that extinct theropods could swim—it would be shocking if a diverse and globally distributed group such as theropods, which lived in many habitats, could not swim! Likewise, no studies have rigourously inferred adept swimming capacity in any but the most specialised taxa (e.g. the loon-like hesperornithiforms). The laterally compressed body forms, small forelimbs (unsuitable for paddling or stabilising the cranial half of the body), distally rigid tails and lack of webbed toes of most extinct theropods rendered them poorly specialised for sustained or manoeuvreable swimming. Nonetheless and perhaps unsurprisingly, traces of swimming locomotion are known for theropods (Coombs 1980; Ezquerra et al. 2007). More detailed studies of swimming locomotion have not been conducted, but then there are no clear questions for theropod swimming performance that are of broader importance either.
Conclusion
Our review has identified a recurrent theme of how false dichotomies can stymie or even mislead research into theropod locomotion, with examples in Table 1. Phylogenetic (Fig. 1) and quantitative approaches show that these views, although perhaps heuristically useful initially, overlook the detailed continuity of form and function in theropod limbs. Avoidance of this over-dichtomization is one path to sustained progress in this field. For example, studies such as Gatesy (1990) and Dial (2003a) exemplify how the gulf between neontology and palaeontology is vanishing, and none too soon. No longer are technological tools limiting the kinds of questions that can be asked—with advances in 3D imaging, computer modelling and simulation, phylogenetics and biomechanical analysis, not to mention new data from detailed, quantitative comparative anatomy of extant (e.g. Smith et al. 2006, 2007) and extinct theropods, a greater limitation is investigator time and expertise. We urge that researchers in this area take up the challenge to dismantle disciplinary walls between experimental and theoretical, anatomical and biomechanical, neontological and palaeontological and other false dichotomies. It is with the collapse of these artificial barriers that a new synthesis should dawn: one in which new discoveries, fossil or experimental become quickly integrated into a richer portrait of the history and mechanisms of theropod locomotion.
As no one scientist is likely to be able to tackle all aspects of the challenges involved, interdisciplinary collaboration will become increasingly important and fruitful. Yet, as most methods have ephemeral benefits, becoming obsolete with new advances, an imperative is to contribute empirical data on form and function in extinct and extant animals, which should have more longevity. In this review, we have briefly covered recent developments in the study of locomotor form and especially function in extant birds. The onus is on palaeobiology researchers to extend their expertise into this domain and vice versa; the life sciences as a whole will benefit.
Researchers need to persist in quantitatively reconstructing the locomotion of theropods (e.g. specialised taxa such as tyrannosaurs and taxa more relevant to the evolution of bird locomotion such as basal deinonychosaurs) at least as a methodological challenge. Can we reduce the considerable quantitative ambiguity about their muscle functions, posture, gait and performance or are we near an impasse? We feel that this remains a fundamental question of interest, but not so much in order to reconstruct their detailed palaeoecology. Rather, it is of value for (1) understanding basic biomechanical constraints on locomotion (e.g. allometry of behaviour; Dial et al. 2008b), (2) contributing to the integration of neontological and palaeontological fields (a ‘one life science’ perspective) and (3) understanding the history of avian form and function, thus enriching our understanding of crown group birds (Prum 2002) and broad trends of dinosaurian evolution (Gauthier 1986; Sereno 1999; Xu et al. 2000, 2003).
We are cynical about the palaeoecological value of reconstructions of locomotor performance in any dinosaurs. Whilst we agree with Paul (2008) and others on anatomical grounds that it is intuitively unlikely that ceratopsians and hadrosaurs could outrun large tyrannosaurs (Hutchinson and Garcia 2002), we see little value in pursuing this issue much more deeply because ‘error bars’ on locomotor performance estimates for taxa of roughly similar size probably would overlap appreciably. The situation could be even worse for comparisons of very similar taxa, e.g. coeval Late Jurassic large Morrison theropods. If morphological differences are ∼5% (e.g. Gatesy and Middleton 1997), yet error bars for locomotor performance estimates using such data ∼50%, how can we make informative comparisons? Perhaps the ambiguity about locomotor performance in extinct taxa will remain so wide that we cannot and perhaps never can identify which were more athletic than others, except in cases of great disparity, in which case qualitative functional morphological comparisons may be sufficient. Consider this in conjunction with ambiguities about environments, metabolic energetics (e.g. endurance), behaviour, selective regimes and other critical factors. Consider also how extremely complex and counterintuitive it can be to judge how evolutionarily important maximal performance such as running speed is in field studies of locomotor performance in extant animals (e.g. Calsbeek and Irschick 2007). We confess that we then view such palaeoecological scenarios as teetering on the brink of fantasy, much as we hope they become possible. However, the path to prove us wrong lies clear: The fossil record and the study of biomechanics are full of surprises, and who can say what rigourous cross-fertilisation may reveal.
Cynicism aside, the study of theropod locomotion exemplifies how palaeobiology has entered a new era since the 1980s. Yet, this bountiful future must continue to confront its most vexing obstacle: the unknowable. The primary question facing this field is how wrong are our reconstructions of theropod locomotion and evolution? All such reconstructions are wrong to some degree. Researchers who do modelling/simulation know this particularly well, as their models by their very nature are never truly accurate, only representational abstractions. ‘Realistic models’ are illusory even for extant taxa. The twin tools of validation and sensitivity analysis are indispensable (and inseparable in our view) but have their limitations (Hutchinson 2006). We predict that it is not long, perhaps 10 years or less, before this field reaches an ‘interpretive asymptote,’ beyond which quantitative assessments of locomotor function in theropods cannot scientifically proceed.
Yet, that asymptote’s boundaries remain blurry, we have not yet sufficiently tested their extent. Two incredibly important, still incomplete ingredients of such testing are empirical data on locomotor mechanisms in extant theropods (and other species, where they establish general principles; Hutchinson and Gatesy 2006) and methodological advances particularly for modelling/simulation (e.g. Gatesy et al. 1999; Hutchinson et al. 2005; Baier et al. 2007; Sellers and Manning 2007).
A theme in this review is that the field has matured to the point of recognition that locomotor function is inherently a quantitative, biomechanical phenomenon. Anatomical data alone are useful for formulating functional hypotheses, but can be insufficient for testing them in much detail as locomotion is simply too complex (levels of neural control, multibody dynamics, soft tissue properties and other constraints lie between morphology and performance, e.g. Lauder 1995; Koehl 1996; Zajac et al. 2002). Regardless of the inevitability or apparent insurmountableness of an interpretive asymptote in extinct theropod locomotion, the potential for amazing new discoveries in the fossil record (e.g. Schweitzer et al. 2005) to redefine knowledge of extinct animals will remain a trump card in the hands of those seeking to circumvent it.
References
Abourachid A (1993) Mechanics of standing in birds: functional explanation of lameness problems in giant turkeys. Br Poult Sci 34:887–898
Abourachid A (2001) Kinematic parameters of terrestrial locomotion in cursorial (ratites), swimming (ducks), and striding birds (quail and guinea fowl). Comp Biochem Physiol A 131:113–119
Alexander RMcN (1976) Estimates of speeds of dinosaurs. Nature 261:129–130
Alexander RMcN (1985) Mechanics of posture and gait of some large dinosaurs. Zool J Linn Soc 83:1–25
Alexander RMcN (1989) Dynamics of dinosaurs and other extinct giants. Columbia Univ Press, New York
Alexander RMcN (1991) Doubts and assumptions in dinosaur mechanics. Interdisc Sci Rev 16:175–181
Alexander RMcN (2002) Tendon elasticity and muscle function. Comp Biochem Physiol A 133:1001–1011
Alexander RMcN (2006) Dinosaur biomechanics. Proc Roy Soc Lond B 273:1849–1855
Alonso PD, Milner AC, Ketcham RA, Cookson MJ, Rowe TB (2004) The avian nature of the brain and inner ear of Archaeopteryx. Nature 430:666–669
Altshuler DL, Dudley R (2002) The ecological and evolutionary interface of hummingbird flight physiology. J Exp Biol 205:2325–2336
Altshuler DL, Dudley R, McGuire JA (2004) Resolution of a paradox: hummingbird flight at high elevation does not come without a cost. Proc Nat Acad Sci 101:17731–17736
Anderson FC, Pandy MG (2001) Static and dynamic optimization solutions for gait are practically equivalent. J Biomech 34:153–161
Azizi E, Brainerd EL, Roberts TJ (2008) Variable gearing in pennate muscles. Proc Natl Acad Sci 105:1745–1750
Baier DB, Gatesy SM, Jenkins FA Jr (2007) A critical ligamentous mechanism in the evolution of avian flight. Nature 445:307–310
Barsbold R, Currie PJ, Myhrvold NP, Osmólska H, Tsogtbaatar K, Watabe M (2000) A pygostyle from a non-avian theropod. Nature 403:155–156
Bates KT, Manning PL, Vila B, Hodgetts D (2008) Three-dimensional modelling and analysis of dinosaur trackways. Palaeontology 51:999–1010
Baumel JJ (1988) Functional morphology of the tail apparatus of the pigeon (Columba livia). Adv Anat Embryol Cell Biol 110:1–115
Biewener AA (1989) Scaling body support in mammals: limb design and muscle mechanics. Science 245:45–48
Biewener AA (1990) Biomechanics of mammalian terrestrial locomotion. Science 250:1097–1103
Biewener AA, Daley MA (2007) Unsteady locomotion: integrating muscle function with whole body dynamics and neuromuscular control. J Exp Biol 210:2949–2960
Biewener AA, Roberts TJ (2000) Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exerc Sport Sci Rev 28:99–107
Blanco RE, Jones WJ (2005) Terror birds on the run: a mechanical model to estimate its maximum running speed. Proc R Soc Lond B 272:1769–1773
Blanco RE, Mazzetta GV (2001) A new approach to evaluate the cursorial ability of the giant theropod Giganotosaurus carolinii. Acta Palaeontol Pol 46:193–202
Bonser RH (1999) Branching out in locomotion: the mechanics of perch use in birds and primates. J Exp Biol 202:1459–1463
Bundle MW, Dial KP (2003) Mechanics of wing-assisted incline running (WAIR). J Exp Biol 206:4553–4564
Bundle MW, Hoppeler H, Vock R, Tester JM, Weyand PG (1999) High metabolic rates in running birds. Nature 397:31–32
Burgers P, Chiappe LM (1999) The wing of Archaeopteryx as a primary thrust generator. Nature 399:60–62
Bybee PJ, Lee AH, Lamm E-T (2006) Sizing the Jurassic theropod Allosaurus: assessing growth strategy and evolution of ontogenetic scaling of limbs. J Morphol 267:347–359
Calsbeek R, Irschick DJ (2007) The quick and the dead: correlational selection on morphology, performance, and habitat use in island lizards. Evolution 61:2493–2503
Carpenter K (2002) Forelimb biomechanics of nonavian theropod dinosaurs in predation. Senckenb Lethaea 82:59–76
Carrano MT (1998) Locomotion in non-avian dinosaurs: integrating data from hindlimb kinematics, in vivo strains, and bone morphology. Paleobiology 24:450–469
Carrano MT (1999) What, if anything, is a cursor? Categories versus continua for determining locomotor habit in mammals and dinosaurs. J Zool Lond 247:29–42
Carrano MT (2000) Homoplasy and the evolution of dinosaur locomotion. Paleobiology 26:489–512
Carrano MT (2001) Implications of limb bone scaling, curvature and eccentricity in mammals and non-avian dinosaurs. J Zool 254:41–55
Carrano MT, Biewener AA (1999) Experimental alteration of limb posture in the chicken (Gallus gallus) and its bearing on the use of birds as analogs for dinosaur locomotion. J Morphol 240:237–249
Carrier DR, Walter RM, Lee DV (2001) Influence of rotational inertia on turning performance of theropod dinosaurs. J Exp Biol 204:3917–3926
Carrano MT, Wilson JA (2001) Taxon distributions and the tetrapod track record. Paleobiology 27:564–582
Chatterjee S (1997) The rise of birds. Johns Hopkins Univ Press, Baltimore
Chatterjee S, Templin RJ (2003) The flight of Archaeopteryx. Naturwissenschaften 90:27–32
Chatterjee S, Templin RJ (2004) Feathered coelurosaurs from China: new light on the arboreal origin of avian flight. In: Currie PJ, Koppelhus EB, Shugar MA, Wright JL (eds) Feathered dragons: studies on the transition from dinosaurs to birds. Indiana Univ Press, Bloomington, pp 251–281
Chatterjee S, Templin RJ (2007) Biplane wing planform and flight performance of the feathered dinosaur Microraptor gui. Proc Nat Acad Sci 104:1576–1580
Chiappe LM (1997) Climbing Archaeopteryx? A response to Yalden. Archaeopteryx 15:109–112
Chiappe LM (2007) Glorified dinosaurs. University of South Wales Press, Sydney
Christiansen P (1998) Strength indicator values of theropod long bones, with comments on limb proportions and cursorial potential. Gaia 15:241–255
Christiansen P (1999) Long bone scaling and limb posture in non-avian theropods: evidence for differential allometry. J Vertebr Paleontol 19:666–680
Christiansen P (2000) Dinosaur biomechanics. In: Paul GS (ed) The Scientific American book of dinosaurs. St Martin’s, New York, pp 64–75
Christiansen P, Bonde N (2002) Limb proportions and avian terrestrial locomotion. J Ornithol 143:356–371
Christiansen P, Bonde N (2004) Body plumage in Archaeopteryx: a review, and new evidence from the Berlin specimen. Comptes Rendus Palevol 3:99–118
Clarke JA, Middleton KM (2008) Mosaicism, modules, and the evolution of birds: results from a Bayesian approach to the study of morphological evolution using discrete character data. Syst Biol 57:185–201
Clarke JA, Zhou Z, Zhang F (2006) Insight into the evolution of avian flight from a new clade of Early Cretaceous ornithurines from China and the morphology of Yixianornis grabaui. J Anat 208:287–308
Codd JR, Manning PL, Norell MA, Perry SF (2008) Avian-like breathing mechanics in maniraptoran dinosaurs. Proc R Soc Lond B 275:157–161
Coombs WP (1978) Theoretical aspects of cursorial adaptations in dinosaurs. Q Rev Biol 3:393–418
Coombs WP (1980) Swimming ability of carnivorous dinosaurs. Science 207:1198–1200
Corr SA, McCorquodale CC, McGovern RE, Gentle MJ, Bennett D (2003) Evaluation of ground reaction forces produced by chickens walking on a force plate. Am J Vet Res 64:76–82
Corr SA, McCorquodale CC, McDonald J, Gentle MJ, McGovern RE (2007) A force plate study of avian gait. J Biomech 40:2037–2043
Daley MA, Biewener AA (2003) Muscle force-length dynamics during level versus incline locomotion: a comparison of in vivo performance of two guinea fowl ankle extensors. J Exp Biol 206:2941–2958
Daley MA, Usherwood JR, Felix G, Biewener AA (2006) Running over rough terrain: guinea fowl maintain dynamic stability despite a large unexpected change in substrate height. J Exp Biol 209:171–187
Daley MA, Felix G, Biewener AA (2007) Running stability is enhanced by a proximo-distal gradient in joint neuromechanical control. J Exp Biol 210:383–394
Day JJ, Norman DB, Upchurch P, Powell HP (2002) Dinosaur locomotion from a new trackway. Nature 415:494–495
de Valais S, Melchor RN (2008) Ichnotaxonomy of bird-like footprints: an example from the Late Triassic–Early Jurassic of northwest Argentina. J Vertebr Paleontol 28:145–159
Dial KP (2003a) Wing-assisted incline running and the origin of flight. Science 299:402–404
Dial KP (2003b) Evolution of avian locomotion: correlates of flight style, locomotor modules, nesting biology, body size, development, and the origin of flapping flight. Auk 120:941–952
Dial KP, Randall RJ, Dial TR (2006) What use is half a wing in the ecology and evolution of flight in birds? Bioscience 56:437–445
Dial KP, Jackson BE, Segre P (2008a) A fundamental avian wing-stroke provides a new perspective on the origin of flight. Nature 451:985–989
Dial KP, Greene E, Irschick DJ (2008b) Allometry of behavior. TREE 23:394–401
Dyke GJ, Norell MA (2005) Caudipteryx as a non-avialan theropod rather than a flightless bird. Acta Palaeontol Pol 50:101–116
Dyke DJ, Nudds RL (2008) The fossil record and limb disparity of enantiornithes, the dominant flying birds of the Cretaceous. Lethaia. doi:10.1111/j.1502-3931.2008.00135.x
Earls KD (2000) Kinematics and mechanics of ground take-off in the starling Sturnis vulgaris and the quail Coturnix coturnix. J Exp Biol 203:725–739
Ellerby DJ, Marsh RL (2006) The energetic costs of trunk and distal-limb loading during walking and running in guinea fowl Numida meleagris: II. Muscle energy use as indicated by blood flow. J Exp Biol 209:2064–2075
Ellerby DJ, Cleary ME, Marsh RL, Buchanan CA (2003) Measurement of maximum oxygen consumption in guinea fowl Numida meleagris indicates that birds and mammals display a similar diversity of aerobic scopes during running. Physiol Biochem Zool 76:695–703
Ellerby DJ, Henry HT, Carr JA, Buchanan CI, Marsh RL (2005) Blood flow in guinea fowl Numida meleagris as an indicator of energy expenditure by individual muscles during walking and running. J Physiol 564:631–648
Erickson GM, Makovicky PJ, Currie PJ, Norell MA, Yerby SA, Brochu CA (2004) Gigantism and comparative life-history parameters of tyrannosaurid dinosaurs. Nature 430:772–775
Ezquerra R, Doublet S, Costeur L, Galton PM, Perez-Lorente F (2007) Were non-avian theropod dinosaurs able to swim? Supportive evidence from an Early Cretaceous trackway, Cameros Basin (La Rioja, Spain). Geology 35:507–510
Farlow JO, Gatesy SM, Holtz TR Jr, Hutchinson JR, Robinson JM (2000) Theropod locomotion. Am Zool 40:640–663
Feduccia A (1993) Evidence from claw geometry indicating arboreal habits of Archaeopteryx. Science 259:790–79
Gabaldón AM, Nelson FE, Roberts TJ (2004) Mechanical function of two ankle extensors in wild turkeys: shifts from energy production to energy absorption during incline versus decline running. J Exp Biol 207:2277–2288
Gabaldón AM, Nelson FE, Roberts TJ (2007) Relative shortening velocity in locomotor muscles: turkey ankle extensors operate at low V/V(max). Am J Physiol Regul Integr Comp Physiol 29:R200–2010
Gao C, Chiappe LM, Meng Q, O’Connor JK, Wang X, Cheng X, Liu J (2008) A new basal lineage of Early Cretaceous birds from China and its implications on the evolution of the avian tail. Palaeontology 51:775–791
Garner JP, Taylor GK, Thomas ALR (1999) On the origins of birds: the sequence of character acquisition in the evolution of avian flight. Proc R Soc Lond 266:1259–1266
Gatesy SM (1990) Caudofemoral musculature and the evolution of theropod locomotion. Paleobiology 16:170–186
Gatesy SM (1995) Functional evolution of the hindlimb and tail from basal theropods to birds. In: Thomason, JJ (ed) Functional morphology in vertebrate paleontology. Cambridge University Press, Cambridge, pp 219–234
Gatesy SM (1999a) Guineafowl hind limb function I: cineradiographic analysis and speed effects. J Morphol 240:115–125
Gatesy SM (1999b) Guineafowl hind limb function II: electromyographic analysis and motor pattern evolution. J Morphol 240:127–142
Gatesy SM (2001a) Skin impressions in Triassic theropod tracks as records of foot movement. Bull Mus Comp Zool 156:137–149
Gatesy SM (2001b) The evolutionary history of the theropod caudal locomotor module. In: Gauthier JA, Gall LF (eds) New perspectives on the origin and early evolution of birds. Peabody Museum of Natural History, New Haven, pp 333–350
Gatesy SM (2002) Locomotor evolution on the line to modern birds. In: Chiappe L, Witmer L (eds) Mesozoic birds: above the heads of dinosaurs. University of California Press, Berkeley, pp 432–447
Gatesy SM (2003) Direct and indirect track features: what sediment did a dinosaur touch? Ichnos 10:91–98
Gatesy SM, Baier DB (2005) The origin of the avian flight stroke: a kinematic and kinetic perspective. Paleobiology 31:382–339
Gatesy SM, Biewener AA (1991) Bipedal locomotion: effects of speed, size and limb posture in birds and humans. J Zool 224:127–147
Gatesy SM, Dial KP (1996) From frond to fan: Archaeopteryx and the evolution of short-tailed birds. Evolution 50:2037–2048
Gatesy SM, Middleton KM (1997) Bipedalism, flight, and the evolution of theropod diversity. J Vertebr Paleontol 17:308–329
Gatesy SM, Middleton KM (2000) Theropod forelimb design and evolution. Zool J Linn Soc 128:149–187
Gatesy SM, Middleton KM (2006) Skeletal adaptations for flight. In: Hall BK (ed) Fins into limbs: evolution, development, and transformation. University of Chicago Press, Chicago, pp 269–283
Gatesy SM, Middleton KM, Jenkins FA Jr, Shubin NH (1999) Three-dimensional preservation of foot movements in Triassic theropod dinosaurs. Nature 399:141–144
Gatesy SM, Shubin NH, Jenkins FA Jr (2005) Anaglyph stereo imaging of dinosaur track morphology and microtopography. Paleontologia Electronica 8.1.10A
Gatesy, SM, Baeker M, Hutchinson JR (2008) Constraint-based exclusion of limb poses for reconstructing theropod dinosaur locomotion. J Vert Paleo (in press)
Gauthier JA (1986) Saurischian monophyly and the origin of birds. Mem Calif Acad Sci 8:1–55
Gauthier JA, Padian K (1985) Phylogenetic, functional, and aerodynamic analyses of the origin of birds and their flight. In: Hecht MK, Ostrom JH, Viohl G, Wellnhofer P (eds) The beginnings of birds. Freunde des Jura-Museums Eichstätt, Eichstätt, Germany, pp 185–197
Gegenbaur C (1864) Untersuchungen zur vergleichenden Anatomie der Wirbelthiere: Erstes Heft. Carpus und Tarsus. Verlag von Wilhelm Engelmann, Leipzig
Genise JF, Melchor RN, Archangelsky M, Bala LO, Straneck R, de Valais S (2008) Application of neoichnological studies to behavioural and taphonomic interpretation of fossil bird-like tracks from lacustrine settings: the Late Triassic–Early Jurassic? Santo Domingo Formation, Argentina. Palaeogeog. doi:10.1016/j.palaeo.2008.08.014
Gishlick AD (2001) The function of the manus and forelimb of Deinonychus antirrhopus and its importance for the origin of avian flight. In: Gauthier JA, Gall LF (eds) New perspectives on the origin and early evolution of birds. Peabody Museum of Natural History, New Haven, pp 301–318
Glen CL, Bennett MB (2007) Foraging modes of birds and non-avian theropods. Curr Biol 17:R911–R912
Goetz JE, Derrick TR, Pedersen DR, Robinson DA, Conzemius MG, Brown TD (2008) Hip joint contact force in the emu (Dromaius novaehollandiae) during normal level walking. J Biomech 41:770–778
Griffin T, Kram R (2000) Penguin waddling is not wasteful. Nature 408:929
Hancock JA, Stevens NJ, Biknevicius AR (2007) Whole-body mechanics and kinematics of terrestrial locomotion in the Elegant-crested Tinamou Eudromia elegans. Ibis 149:605–614
Hedenström A (2002) Aerodynamics, evolution and ecology of avian flight. Trends Ecol Evol 17:415–422
Hedenström A, Griethuijsen LV, Rosen M, Spedding GR (2006) Vortex wakes of birds: recent developments using digital particle image velocimetry in a wind tunnel. Anim Biol 56:535–549
Hedrick TL, Tobalske BW, Biewener AA (2003) How cockatiels (Nymphicus hollandicus) modulate pectoralis power output across flight speeds. J Exp Biol 207:1689–1702
Henderson D (2003) Footprints, trackways, and hip heights of bipedal dinosaurs—testing hip height predictions with computer models. Ichnos 10:99–114
Henderson D (2006) Simulated weathering of dinosaur tracks and the implications for their characterization. Can J Earth Sci 43:691–704
Henderson D, Snively E (2003) Tyrannosaurus en pointe: allometry minimized rotational inertia of large carnivorous dinosaurs. Proc Roy Soc Lond B 271:57–60
Henry HT, Ellerby DJ, Marsh RL (2005) Performance of guinea fowl Numida meleagris during jumping requires storage and release of elastic energy. J Exp Biol 208:3293–3302
Hertel F, Campbell KE Jr (2007) The antitrochanter of birds: form and function in balance. Auk 124:789–805
Higham TE, Biewener AA (2008) Integration within and between muscles during terrestrial locomotion: effects of incline and speed. J Exp Biol 211:2303–2316
Higham TE, Nelson FE (2008) The integration of lateral gastrocnemius muscle function and kinematics in running turkeys. Zoology 6:483–493
Higham TE, Biewener AA, Wakeling JM (2008) Functional diversification within and between muscle synergists during locomotion. Biol Lett 4:41–44
Holtz TR Jr (1995) The arctometatarsalian pes, an unusual structure of the metatarsus of Cretaceous Theropoda (Dinosauria: Saurischia). J Vertebr Paleontol 14:480–519
Hopson JA (2001) Ecomorphology of avian and nonavian theropod phalangeal proportions: implications for the arboreal versus terrestrial origin of bird flight. In: Gauthier JA, Gall LF (eds) New perspectives on the origin and early evolution of birds. Peabody Museum of Natural History, New Haven, pp 210–235
Hutchinson JR (2001a) The evolution of pelvic osteology and soft tissues on the line to extant birds (Neornithes). Zool J Linn Soc 131:123–168
Hutchinson JR (2001b) The evolution of femoral osteology and soft tissues on the line to extant birds (Neornithes). Zool J Linn Soc 131:169–197
Hutchinson JR (2002) The evolution of hindlimb tendons and muscles on the line to crown-group birds. Comp Biochem Physiol A 133:1051–1086
Hutchinson JR (2003) Biomechanics: early birds surmount steep slopes. Nature 426:777–778
Hutchinson JR (2004a) Biomechanical modeling and sensitivity analysis of bipedal running ability. I. Extant taxa. J Morphol 262:421–440
Hutchinson JR (2004b) Biomechanical modeling and sensitivity analysis of bipedal running ability. II. Extinct taxa. J Morphol 262:441–461
Hutchinson JR (2006) The evolution of archosaur locomotion. Comptes Rendus Palevol 5:519–530
Hutchinson JR, Gatesy SM (2000) Adductors, abductors, and the evolution of archosaur locomotion. Paleobiology 26:734–751
Hutchinson JR, Garcia M (2002) Tyrannosaurus was not a fast runner. Nature 415:1018–1021
Hutchinson JR, Gatesy SM (2006) Dinosaur locomotion: beyond the bones. Nature 440:292–294
Hutchinson JR, Anderson FC, Blemker S, Delp SL (2005) Analysis of hindlimb muscle moment arms in Tyrannosaurus rex using a three-dimensional musculoskeletal computer model. Paleobiology 31:676–701
Hutchinson JR, Ng-Thow-Hing V, Anderson FC (2007) A 3D interactive method for estimating body segmental parameters in animals: application to the turning and running performance of Tyrannosaurus rex. J Theor Biol 246:660–680
Hutchinson JR, Miller CE, Fritsch G, Hildebrandt T (2008) The anatomical foundation for multidisciplinary studies of animal limb function: examples from dinosaur and elephant limb imaging studies. In: Frey R, Endo H (eds) Anatomical imaging: towards a new morphology. Springer, Tokyo, pp 23–38
Huxley TH (1868) On the animals which are most nearly intermediate between birds and reptiles. Geol Mag 5:357–365
Jasinoski SC, Russell AP, Currie PJ (2006) An integrative phylogenetic and extrapolatory approach to the reconstruction of dromaeosaur (Theropoda: Eumaniraptora) shoulder musculature. Zool J Linn Soc 146:301–344
Jenkins FA Jr (1993) The evolution of the avian shoulder joint. Am J Sci 293–A:253–267
Jindrich DL, Smith NC, Jespers K, Wilson AM (2007) Mechanics of cutting maneuvers by ostriches (Struthio camelus). J Exp Biol 210:1378–1390
Jones TD, Farlow JO, Ruben JA, Henderson DM, Hillenius WJ (2000) Cursoriality in bipedal archosaurs. Nature 406:716–718
Kaiser GW (2000) Alternative origins for flight in birds. N Jb Geol Palaeont Abh 217:27–39
Kim JY, Kim KS, Lockley MG, Yang SY, Seo SJ, Choi HI, Lim JD (2008) New didactyl dinosaur footprints (Dromaeosauripus hamanensis ichnogen. et ichnosp. nov.) from the Early Cretaceous Haman Formation, south coast of Korea. Palaeogeogr Palaeoclimatol Palaeoecol 262:72–78
Koehl MAR (1996) When does morphology matter? Ann Rev Ecolog Syst 27:501–542
Kuo A (1999) Stabilization of lateral motion in passive dynamic walking. Int J Rob Res 18:917–930
Kurz MJ, Scott-Pandorf M, Arellano C, Olsen D, Whitaker G (2008) The penguin waddling gait pattern has a more consistent step width than step length. J Theor Biol 252:272–276
Lauder GV (1995) On the inference of function from structure. In: Thomason JJ (ed) Functional morphology in vertebrate paleontology. Cambridge Univ Press, Cambridge, pp 1–15
Li R, Lockley MG, Makovicky PJ, Matsukawa M, Norell MA, Harris JD, Liu M (2008) Behavioral and faunal implications of Early Cretaceous deinonychosaur trackways from China. Naturwissenschaften 95:185–191
Lipkin C, Carpenter K (2008) Looking Again at the Forelimb of Tyrannosaurus rex. In: Larson PL, Carpenter K (eds) Tyrannosaurus rex: the tyrant king. Indiana Univ Press, Bloomington, pp 167–192
Lockley MG, Hunt AP, Moratalla JJ, Matsukawa M (1994) Limping dinosaurs? Trackway evidence for abnormal gaits. Ichnos 3:193–202
Lockley MG, Li R, Harris JD, Matsukawa M, Liu M (2007) Earliest zygodactyl bird feet: evidence from early Cretaceous roadrunner-like tracks. Naturwissenschaften 94:657–665
Long CA, Zhang GP, George TF (2002) Physical and evolutionary problems in take-off runs of bipedal winged vertebrates. Archaeopteryx 20:63–71
Long CA, Zhang GP, George TF, Long CF (2003) Physical theory, origin of flight, and a synthesis proposed for birds. J Theor Biol 224:9–26
Longrich N (2006) Structure and function of hindlimb feathers in Archaeopteryx lithographica. Paleobiology 32:417–431
Ma MS, Ma WY, Nieuwland I, Easley RR (2002) Why Archaeopteryx did not run over water. Archaeopteryx 20:51–56
Main RP, Biewener AA (2007) Skeletal strain patterns and growth in the emu hindlimb during ontogeny. J Exp Biol 210:2676–2690
Manning PL (2004) A new approach to the analysis and interpretation of tracks: examples from the dinosauria. Geol Soc Lond Spec Pub 228:93–123
Manning PL (2008) T. rex speed trap. In: Larson PL, Carpenter K (eds) Tyrannosaurus rex: the tyrant king. Indiana Univ Press, Bloomington, pp 205–231
Manning PL, Payne D, Pennicott J, Barrett PM, Ennos RA (2008) Dinosaur killer claws or climbing crampons? Biol Lett 2:110–112
Marsh RL, Ellerby DJ (2006) Partitioning locomotor energy use among and within muscles: muscle blood flow as a measure of muscle oxygen consumption. J Exp Biol 209:2385–2394
Marsh RL, Ellerby DJ, Henry HT, Carr JA, Buchanan CA (2004) Partitioning the energetics of walking and running: swinging the limbs is expensive. Science 303:80–83
Marsh RL, Ellerby DJ, Henry HT, Rubenson J (2006) The energetic costs of trunk and distal-limb loading during walking and running in guinea fowl Numida meleagris: I. Organismal metabolism and biomechanics. J Exp Biol 209:2050–2063
Mayr G, Pohl B, Peters DS (2005) A well-preserved Archaeopteryx specimen with theropod features. Science 310:1483–1486
Mayr G, Pohl B, Hartman S, Peters DS (2007) The tenth skeletal specimen of Archaeopteryx. Zool J Linn Soc 149:97–116
Mazzetta GV, Fariña RA, Viscaino SF (1998) On the paleobiology of the South American horned theropod Carnotaurus sastrei Bonaparte. Gaia 15:185–192
Mazzetta GV, Christiansen P, Fariña RA (2004) Giants and bizarres: body size of some southern South American Cretaceous dinosaurs. Hist Biol 16:71–83
McGowan CP, Duarte HA, Main JB, Biewener AA (2006) Effects of load carrying on metabolic cost and hindlimb muscle dynamics in guinea fowl (Numida meleagris). J Appl Physiol 101:1060–1069
Melchor RN, de Valais S, Genise JF (2002) Bird-like fossil footprints from the Late Triassic. Nature 417:936–938
Middleton KM (2001) The morphological basis of hallucal orientation in extant birds. J Morphol 250:51–60
Middleton KM, Gatesy SM (2000) Theropod forelimb design and evolution. Zool J Linn Soc 128:149–187
Milan J (2006) Variations in the morphology of emu (Dromaius novaehollandiae) tracks reflecting differences in walking pattern and substrate consistency: ichnotaxonomic implications. Palaeontology 49:405–420
Milan J, Bromley R (2006) True tracks, undertracks and eroded tracks, experimental work with tetrapod tracks in laboratory and field. Palaeogeogr Palaeoclimatol Palaeoecol 231:253–264
Milan J, Bromley R (2008) The impact of sediment consistency on track and undertrack morphology: experiments with emu tracks in layered cement. Ichnos 15:18–24
Milan J, Loope DB (2007) Preservation and erosion of theropod tracks in eolian deposits: examples from the Middle Jurassic Entrada Sandstone, Utah, U.S.A. J Geol 115:375–386
Milan J, Clemmensen LB, Bonde N (2004) Vertical sections through dinosaur tracks (Late Triassic lake deposits, East Greenland)—undertracks and other subsurface deformations revealed. Lethaia 37:285–296
Moreno K, Carrano MT, Snyder R (2007) Morphological changes in pedal phalanges through ornithopod dinosaur evolution: a biomechanical approach. J Morphol 268:50–63
Mossman DJ, Brüning R, Powell H (2003) Anatomy of a Jurassic theropod trackway from Ardley, Oxfordshire, UK. Ichnos 10:195–207
Naish D (2000) Theropod dinosaurs in the trees: a historical review of arboreal habits amongst nonavian theropods. Archaeopteryx 18:35–41
Nelson FE, Roberts TJ (2008) Task-dependent force sharing between muscle synergists during locomotion in turkeys. J Exp Biol 211:1211–1220
Nelson FE, Gabaldón AM, Roberts TJ (2004) Force–velocity properties of two avian hindlimb muscles. Comp Biochem Physiol A 137:711–721
Novas FE (1996) Dinosaur monophyly. J Vertebr Paleontol 16:723–741
Nudds RL, Dyke GJ, Rayner JMV (2004) Forelimb proportions and the evolutionary radiation of Neornithes. Proc R Soc Lond B 271:324–327
Padian K (2001) Stages in the origin of bird flight: beyond the arboreal-cursorial dichotomy. In: Gauthier JA, Gall LF (eds) New perspectives on the origin and early evolution of birds. Peabody Museum of Natural History, New Haven, pp 255–272
Padian K (2003) Four-winged dinosaurs, bird precursors, or neither? Bioscience 53:450–452
Padian K, Dial KP (2005) Could four-winged dinosaurs fly? Nature 438:E3
Paul GS (1988) Predatory dinosaurs of the world. Simon and Schuster, New York
Paul GS (1998) Limb design, function and running performance in ostrich-mimics and tyrannosaurs. Gaia 15:257–270
Paul GS (2002) Dinosaurs of the air: the evolution and loss of flight in dinosaurs and birds. Johns Hopkins Univ Press, Baltimore
Paul GS (2005) Body and tail posture in theropod dinosaurs. In: Carpenter K (ed) The carnivorous dinosaurs. Indiana Univ Press, Bloomington, pp 238–246
Paul GS (2008) The extreme lifestyle and habits of the gigantic tyrannosaurid superpredators of the late Cretaceous of North America and Asia. In: Larson PL, Carpenter K (eds) Tyrannosaurus rex: the tyrant king. Indiana Univ Press, Bloomington, pp 307–351
Peters DS, Görgner E (1992) A comparative study on the claws of Archaeopteryx. LA Co Mus Sci Ser 36:29–37
Pike AVL, Maitland DP (2004) Scaling of bird claws. J Zool 26:73–81
Platt BF, Hasiotis ST (2008) A new system for describing and classifying tetrapod tail traces with implications for interpreting the dinosaur tail trace record. Palaios 23:3–13
Pontzer H, Lieberman DE, Momin E, Devlin MJ, Polk JD, Hallgrímsson B, Cooper DM (2006) Trabecular bone in the bird knee responds with high sensitivity to changes in load orientation. J Exp Biol 209:57–65
Prum RO (2002) Why ornithologists should care about the theropod origin of birds. Auk 119:1–17
Reilly SM (2000) Locomotion in the quail (Coturnix japonica): the kinematics of walking and increasing speed. J Morphol 243:173–185
Ren L, Butler M, Miller C, Schwerda D, Fischer M, Hutchinson JR (2008) The movements of limb segments and joints during locomotion in African and Asian elephants. J Exp Biol 211:2735–2751
Roberts TJ (2001) Muscle force and stress during running in dogs and wild turkeys. Bull Mus Comp Zool 156:283–295
Roberts TJ (2002) The integrated function of muscles and tendons during locomotion. Comp Biochem Physiol A 133:1087–1099
Roberts TJ, Scales JA (2002) Mechanical power output during running accelerations in wild turkeys. J Exp Biol 205:1485–1494
Roberts TJ, Kram R, Weyand PG, Taylor CR (1998a) Energetics of bipedal running. I. Metabolic cost of generating force. J Exp Biol 201:2745–2751
Roberts TJ, Chen MS, Taylor CR (1998b) Energetics of bipedal running. II. Limb design and running mechanics. J Exp Biol 201:2753–2762
Roberts TJ, Higginson BK, Nelson FE, Gabaldón AM (2007) Muscle strain is modulated more with running slope than speed in wild turkey knee and hip extensors. J Exp Biol 210:2510–2517
Rubenson J, Heliams DB, Lloyd DG, Fournier PA (2003) Gait selection in the ostrich: mechanical and metabolic characteristics of walking and running with and without an aerial phase. Proc R Soc Lond B 271:1091–1099
Rubenson J, Henry HT, Dimoulas PM, Marsh RL (2006) The cost of running uphill: linking organismal and muscle energy use in guinea fowl (Numida meleagris). J Exp Biol 209:2395–2408
Rubenson J, Lloyd DG, Besier TF, Heliams DB, Fournier PA (2007) Running in ostriches (Struthio camelus): three-dimensional joint axes alignment and joint kinematics. J Exp Biol 210:2548–2562
Sans JL, Alvarez JC, Soriano C, Hernandez-Carrasquilla F, Perez-Moreno BP, Meseguer J (2002) Wing loading in primitive birds. In: Zhou Z, Zhang F (eds) Proceedings of the 5th Symposium of the Society of Avian Paleontology and Evolution. Science, Beijing, pp 253–258
Schweitzer MH, Wittmeyer JL, Horner JR, Toporski JK (2005) Soft-tissue vessels and cellular preservation in Tyrannosaurus rex. Science 307:1952–1955
Scovil C, Ronsky J (2006) Sensitivity of a Hill-based muscle model to perturbations in model parameters. J Biomech 39:2055–2063
Sellers WI, Manning PM (2007) Estimating dinosaur maximum running speeds using evolutionary robotics. Proc R Soc Lond B 274:2711–2716
Senter P (2005) Function in the stunted forelimbs of Mononykus olecranus (Theropoda), a dinosaurian anteater. Paleobiology 31:373–381
Senter P (2006a) Forelimb function in Ornitholestes hermanni Osborn (Dinosauria, Theropoda). Palaeontology 49:1029–1034
Senter P (2006b) Comparison of forelimb function between Deinonychus and Bambiraptor (Theropoda: Dromaeosauridae). J Vertebr Paleontol 26:897–906
Senter P (2006c) Scapular orientation in theropods and basal birds, and the origin of flapping flight. Acta Palaeontol Pol 51:305–313
Senter P (2007) A new look at the phylogeny of Coelurosauria (Dinosauria: Theropoda). J Systemat Paleo 5:429–463
Senter P, Parrish JM (2005) Functional analysis of the hands of the theropod dinosaur Chirostenotes pergracilis: evidence for an unusual paleoecological role. PaleoBios 25:9–19
Senter P, Parrish JM (2006) Forelimb function in the theropod dinosaur Carnotaurus sastrei, and its behavioral implications. PaleoBios 26:7–17
Senter P, Robins JH (2005) Range of motion in the forelimb of the theropod dinosaur Acrocanthosaurus atokensis, and implications for predatory behaviour. J Zool 266:307–318
Sereno PC (1999) The evolution of dinosaurs. Science 284:2137–2147
Smith NC, Wilson AM, Jespers KJ, Payne RC (2006) Muscle architecture and functional anatomy of the pelvic limb of the ostrich (Struthio camelus). J Anat 209:765–777
Smith NC, Payne RC, Jespers KJ, Wilson AM (2007) Muscle moment arms of pelvic limb muscles of the ostrich (Struthio camelus). J Anat 211:313–324
Snively E, Russell AP (2002) The tyrannosaurid metatarsus: bone strain and inferred ligament function. Senckenb Lethaea 82:35–42
Snively E, Russell AP (2003) Kinematic model of tyrannosaurid (Dinosauria: Theropoda) arctometatarsus function. J Morphol 255:215–227
Snively E, Russell AP, Powell GL (2004) Evolutionary morphology of the coelurosaurian arctometatarsus: descriptive, morphometric and phylogenetic approaches. Zool J Linn Soc 142:525–553
Spedding GR, Rosen M, Hedenstrom A (2003) A family of vortex wakes generated by a thrush nightingale in free flight in a wind tunnel over its entire natural range of speeds. J Exp Biol 206:2313–2344
Stevens KA, Larson P, Wills ED, Anderson A (2008) Rex, sit: digital modeling of Tyrannosaurus rex at rest. In: Larson PL, Carpenter K (eds) Tyrannosaurus rex: the tyrant king. Indiana Univ Press, Bloomington, pp 193–203
Summers A (2003) Uphill flight. Nat Hist Dec 2003/Jan 2004:30–31
Thulborn T (1990) Dinosaur tracks. Chapman, London
Tobalske BW (2007) Biomechanics of bird flight. J Exp Biol 210:3135–3146
Tobalske BW, Dial KP (2007) Aerodynamics of wing-assisted incline running in birds. J Exp Biol 210:1742–1751
Tobalske BW, Hedrick TL, Dial KP, Biewener AA (2003) Comparative power curves in bird flight. Nature 421:363–366
Usherwood JR, Wilson AM (2005a) No force limit on greyhound sprint speed. Nature 438:753–754
Usherwood JR, Wilson AM (2005b) Accounting for elite indoor 200m sprint results. Biol Lett 2:47–50
Usherwood JR, Hedrick TL, Biewener AA (2003) The aerodynamics of avian take-off from direct pressure measurements in Canada geese (Branta canadensis). J Exp Biol 206:4051–4056
Usherwood JR, Hedrick TL, McGowan CP, Biewener AA (2005) Direct pressure maps for wings and tails of pigeons in slow, flapping flight, and their energetic implications. J Exp Biol 208:355–369
Usherwood JR, Szymanek KL, Daley MA (2008) Compass gait mechanics account for top walking speeds in ducks and humans. J Exp Biol 211:3744–3749
Videler JJ (2000) Archaeopteryx: A dinosaur running over water? Archaeopteryx 18:27–34
Videler JJ (2006) Avian flight. Oxford Univ Press, Oxford
Warrick DR, Tobalske BW, Powers DP (2005) Aerodynamics of the hovering hummingbird. Nature 435:1094–1097
Weyand PG, Sternlight DB, Bellizzi MJ, Wright SJ (2000) Faster top running speeds are achieved with greater ground forces not more rapid leg movements. J Appl Physiol 89:1991–1999
Witmer LM (1995) The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In: Thomason JJ (ed) Functional morphology in vertebrate paleontology. Cambridge Univ Press, Cambridge, pp 19–33
Witmer LM (2002) The debate on avian ancestry: phylogeny, function, and fossils. In: Chiappe LM, Witmer LM (eds) Mesozoic birds: above the heads of dinosaurs. University of California Press, Berkeley, pp 3–30
Xu X, Zhang F (2005a) A new maniraptoran dinosaur from China with long feathers on the metatarsus. Naturwissenschaften 92:173–177
Xu X, Zhang F (2005b) A new maniraptoran dinosaur with long feathers on the metatarsus. Naturwissenschaften 92:173–177
Xu X, Zhou Z, Wang X (2000) The smallest known non-avian theropod. Nature 408:705–708
Xu X, Zhou Z, Wang X, Kuang X, Zhang F, Du X (2003) Four-winged dinosaurs from China. Nature 421:335–340, http://www.nature.com/nature/journal/v421/n6921/abs/nature01342.html-a1
Xu X, Zhou Z, Wang X, Kuang X, Zhjang F, Du X (2005) Reply to Padian et al. (2005). Nature 438:E3–E4
Xu X, Tan Q, Wang J, Zhao X, Tan L (2007) A gigantic bird-like dinosaur from the Late Cretaceous of China. Nature 447:844–847
Yalden DW (1997) Climbing Archaeopteryx. Archaeopteryx 15:107–108
Zajac FE, Neptune RR, Kautz SA (2002) Biomechanics and muscle coordination of human walking Part I: Introduction to concepts, power transfer, dynamics and simulations. Gait Posture 16:215–232
Zeffer A, Norberg UML (2003) Leg morphology and locomotion in birds: requirements for force and speed during ankle flexion. J Exp Biol 206:1085–1097
Zhang F, Zhou Z, Xu X, Wang X (2002) A juvenile coelurosaurian theropod from China indicates arboreal habits. Naturwissenschaften 89:394–398
Zhang F, Zhou Z, Dyke G (2006) Feathers and ‘feather-like’ integumentary structures in Liaoning birds and dinosaurs. Geol J 41:395–401
Zhang F, Zhou Z, Xu X, Wang X, Sullivan C (2008) A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature 455:1105–1108
Zheng X, Zhang Z, Hou L (2007) A new enantiornitine Enantiornitine bird with four long retrices from the Early Cretaceous of Northern Hebei, China. Acta Geol Sin 81:703–708
Zhou Z (2004) The origin and early evolution of birds: discoveries, disputes, and perspectives from fossil evidence. Naturwissenschaften 91:455–471
Zhou Z, Farlow JO (2001) Flight capability and habits of Confuciusornis. In: Gauthier JA, Gall LF (eds) New perspectives on the origin and early evolution of birds. Peabody Museum of Natural History, New Haven, pp 237–254
Zhou Z, Zhang F (2003) Jeholornis compared to Archaeopteryx, with a new understanding of the earliest avian evolution. Naturwissenschaften 90:220–225
Zhou Z, Zhang F (2004) Discovery of an ornithurine bird and its implication for Early Cretaceous avian radiation. Proc Nat Acad Sci 102:18998–19002
Acknowledgements
Hearty thanks are due to Eric Snively for lending us the phrase ‘interpretive asymptote.’ We thank the Department of Veterinary Basic Sciences at The Royal Veterinary College for supporting this work. Vivian Allen’s research was partly supported by a Sam and Doris Welles award from the University of California Museum of Paleontology. We appreciate the influence of fellow members of the Structure and Motion Laboratory, Stanford University’s Neuromuscular Biomechanics Laboratory, and Padian Lab at Berkeley. Martin Baeker, Charlotte Miller, Heather Paxton and three anonymous reviewers are thanked for their constructive feedback on earlier drafts of this manuscript. Scott Hartman, Jim Robins and Frederik Spindler are thanked for provision of their artwork as noted in the figure captions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hutchinson, J.R., Allen, V. The evolutionary continuum of limb function from early theropods to birds. Naturwissenschaften 96, 423–448 (2009). https://doi.org/10.1007/s00114-008-0488-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-008-0488-3