Abstract
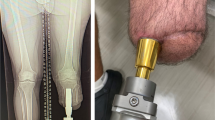
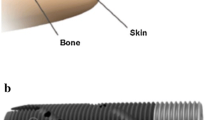
Amputation is an unfortunate outcome of a variety of orthopedic conditions. Many amputees can be functionally fitted with conventional suspension sockets. A substantial subset, however, fails this conventional treatment and is unable to function. In Europe, an alternative to socket-based prostheses has been available for 25 years. Patients there who are unable to functionally use socket-based prostheses have been offered the possibility for transcutaneous osseointegration. With this technology, the prosthetic limb can be rigidly attached to the residual bone, and the socket is eliminated, in many cases enabling improved function and patient satisfaction. In the United States, regulatory barriers have greatly limited the adoption and acceptance of transdermal osseointegration. The Compress® device was developed as an alternate means of fixation for massive endoprostheses, such as distal femoral replacements. A uniquely designed prosthesis is rigidly anchored to the end of the cortical bone and is then subjected to a large axial stress. The bone then grows avidly into the device, providing permanent osseointegration. We have recently adopted this device for transcutaneous use. These procedures have been performed in the United States on a custom regulatory basis. Results of this have been encouraging, and we are planning to begin a regulatory trial in the near future.
Zusammenfassung
Die Amputation ist bedauerliches Ergebnis einer Anzahl orthopädischer Erkrankungen. Viele Patienten können nach Amputation mit herkömmlichen Schaftprothesen funktionell ausgestattet werden. Bei einem wesentlichen Anteil aber gelingt die funktionelle Versorgung mit dieser herkömmlichen Therapie nicht. In Europa ist seit 25 Jahren eine Alternative zur Schaftprothese verfügbar. Patienten, bei denen die funktionelle Verwendung einer Schaftprothese nicht möglich ist, wurde die Option einer transkutanen Osseointegration angeboten. Mit diesem Verfahren kann die Extremitätenprothese fest an dem verbliebenen Knochen verankert werden, und der Schaft wird weggelassen, was in vielen Fällen zu einer besseren Funktion und größeren Patientenzufriedenheit führt. In den Vereinigten Staaten haben regulatorische Hürden die Einführung und Akzeptanz der transdermalen Osseointegration weitgehend beschränkt. Die Compress®-Prothese wurde als alternative Methode zur Fixierung großer Endoprothesen entwickelt, z. B. für den Ersatz des distalen Femurs. Eine speziell angefertigte Prothese wird fest im Ende des kortikalen Knochens verankert und dann einer großen axialen Belastung ausgesetzt. Der Knochen wächst somit rege in das Implantat ein, was zu einer dauerhaften Knochenverankerung führt. Vor Kurzem wurde dieses System für den transkutanen Einsatz angepasst. Diese Operationen sind in den Vereinigten Staaten jeweils auf einer individuellen regulatorischen Grundlage durchgeführt worden. Die Ergebnisse waren ermutigend, und die Autoren planen, in naher Zukunft mit einer Zulassungsstudie anzufangen.





Similar content being viewed by others
References
Al Muderis M, Khemka A, Lord SJ et al (2016) Safety of osseointegrated implants for transfemoral amputees: A two-center prospective cohort study. J Bone Jt Surg 98:900–909
Aschoff HH, Clausen A, Hoffmeister T (2009) Die Endo-Exo-Femurprothese – ein neues Konzept zur Knochengefuhren, prothetischen Versorgung von oberschenkelamputierten Patienten. Z Orthop Unfall 147:610–615
Aschoff HH, Kennon RE, Keggi JM et al (2010) Transcutaneous, distal femoral, intramedullary attachment for above-the-knee prostheses: An endo-exo device. J Bone Jt Surg 92(Suppl 2):180–186
Aschoff HH, Juhnke DL (2012) Evaluation of 10 years-experience with endo-exo femur prostheses – background, data, and results. Z Orthop Unfall 150:607–614
Calvert GT, Cummings JE, Bowles AJ et al (2014) A dual-center review of compressive osseointegration for fixation of massive endoprosthetics: 2‑ to 9‑year followup. Clin Orthop Relat Res 472:822–829
Goldman LH, Morse LJ, O’Donnell RJ, Wustrack RL (2016) How often does spindle failure occur in compressive Osseointegration Endoprostheses for oncologic reconstruction? Clin Orthop Relat Res 474:1714–1723
Hagberg K, Haggstrom E, Uden M et al (2005) Socket versus bone-anchored trans-femoral prostheses: Hip range of motion and sitting comfort. Pros Orthot Int 29:153–163
Hagbeg K, Branemark R, Gunterberg B et al (2008) Osseointegrated trans-femoral amputation prostheses: Prospective results of general and condition-specific quality of life in 18 patients at 2 year follow-up. Prosth Orthot Int 32:29–41
Hanselman AE, Boukhemis KW, Lalli TAJ, Lindsey BA (2017) Osseointegrated transcutaneous device for amputees: A large animal model. J Orthop Res (In submission)
Leijendekkers RA, van Hinte G, Frölke JP et al (2016) Comparison of bone-anchored prostheses and socket prostheses for patients with a lower extremity amputation: A systematic review. Disabil Rehabil (Epub ahead of print)
Levy SW (1995) Amputees: Skin problems and prostheses. Cutis 55:297–301
Lyon CC, Kulkarni J, Zimerson E et al (2000) Skin disorders in amputees. J Am Acad Dermatol 42:501–507
McGough RL. Personal interviews of greater than twenty transcutaneous osseointegrated amputees using four different reconstruction systems
Monument MJ, Bernthal NM, Bowles AJ et al (2015) What are the 5‑year survivorship outcomes of compressive endoprosthetic osseointegration fixation of the femur? Clin Orthop Relat Res 473:883–890
Van de Meent H, Hopman MT, Frolke JP (2013) Walking ability and quality of life in subjects with transfemoral amputation: A comparison of osseointegration with socket prostheses. Arch Phys Med Rehabil 94:2174–2178
van Eck C, McGough RL (2015) Clinical outcome of osseointegrated prostheses for lower extremity amputations: A systematic review of literature. Curr Orthop Prac 26:349–357
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. L. McGough, M. A. Goodman, R. L. Randall, and B. Lindsey are consultants for Zimmer Biomet. J. A. Forsberg, and B. K. Potter declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Redaktion
H. Aschoff, Hannover
Rights and permissions
About this article
Cite this article
McGough, R.L., Goodman, M.A., Randall, R.L. et al. The Compress® transcutaneous implant for rehabilitation following limb amputation. Unfallchirurg 120, 300–305 (2017). https://doi.org/10.1007/s00113-017-0339-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00113-017-0339-9