Abstract
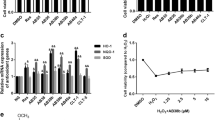
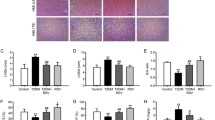
Renal interstitial fibrosis is a major pathologic feature of diabetic nephropathy, while the pathogenesis and therapeutic interventions of diabetic renal interstitial fibrosis are not well established. In this study, we first demonstrated that high glucose could induce renal fibroblast (NRK-49F) cell proliferation and activation to myofibroblasts, accompanied by a significant increase in the intracellular levels of reactive oxygen species (ROS) derived from nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4). ROS-mediated ERK1/2 activation was found to play a crucial role in high glucose-induced fibroblast proliferation and activation. Resveratrol, like the NOX4-targeting small interfering RNA (siRNA), markedly inhibited high glucose-induced fibroblast proliferation and activation by reducing NOX4-derived ROS production. It was then revealed that the increase in the expression of NOX4 induced by high glucose was due to the inactivation of AMP-activated protein kinase (AMPK), which could be reversed by resveratrol. Further in vivo investigation demonstrated that resveratrol treatment significantly attenuated renal fibrosis in db/db mice, accompanied by an evident increase in phospho-AMPK and decrease in NOX4. In summary, our results suggest that high glucose can directly promote renal fibroblasts proliferation and activation in a ROS-dependent manner, and resveratrol is a potential therapeutic agent against diabetic renal fibrosis via regulation of AMPK/NOX4/ROS signaling.
Key message
-
Resveratrol inhibits high glucose-induced NRK cell activation by decreasing NOX4-derived ROS.
-
Resveratrol inhibits high glucose-induced NOX4 expression in NRK cells via activation of AMPK.
-
ROS-activated ERK1/2 signaling is involved in high glucose-induced NRK cell activation.
-
Resveratrol attenuated renal fibrosis in db/db mice via regulation of AMPK/NOX4/ROS signaling.








Similar content being viewed by others
References
Strutz F, Zeisberg M (2006) Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol 17(11):2992–2998
Grande MT, Lopez-Novoa JM (2009) Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat Rev Nephrol 5(6):319–328
LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R (2013) Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19(8):1047–1053
Asada N, Takase M, Nakamura J, Oguchi A, Asada M, Suzuki N, Yamamura K, Nagoshi N, Shibata S, Rao TN et al (2011) Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest 121(10):3981–3990
Manickam N, Patel M, Griendling KK, Gorin Y, Barnes JL (2014) RhoA/Rho kinase mediates TGF-beta1-induced kidney myofibroblast activation through Poldip2/Nox4-derived reactive oxygen species. Am J Physiol Renal Physiol 307(2):F159–F171
Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, Barnes JL (2010) NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol 21(1):93–102
Cheng TH, Cheng PY, Shih NL, Chen IB, Wang DL, Chen JJ (2003) Involvement of reactive oxygen species in angiotensin II-induced endothelin-1 gene expression in rat cardiac fibroblasts. J Am Coll Cardiol 42(10):1845–1854
He T, Guan X, Wang S, Xiao T, Yang K, Xu X, Wang J, Zhao J (2015) Resveratrol prevents high glucose-induced epithelial-mesenchymal transition in renal tubular epithelial cells by inhibiting NADPH oxidase/ROS/ERK pathway. Mol Cell Endocrinol 402:13–20
Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE (2005) Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280(47):39616–39626
Eid AA, Ford BM, Bhandary B, de Cassia Cavaglieri R, Block K, Barnes JL, Gorin Y, Choudhury GG, Abboud HE (2013) Mammalian target of rapamycin regulates Nox4-mediated podocyte depletion in diabetic renal injury. Diabetes 62(8):2935–2947
Holterman CE, Read NC, Kennedy CR (2015) Nox and renal disease. Clin Sci (Lond) 128(8):465–481
Li JM, Shah AM (2003) ROS generation by nonphagocytic NADPH oxidase: potential relevance in diabetic nephropathy. J Am Soc Nephrol 14(8 Suppl 3):S221–S226
Kim WH, Lee JW, Suh YH, Lee HJ, Lee SH, Oh YK, Gao B, Jung MH (2007) AICAR potentiates ROS production induced by chronic high glucose: roles of AMPK in pancreatic beta-cell apoptosis. Cell Signal 19(4):791–805
Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT et al (2006) Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 55(8):2256–2264
Motoshima H, Goldstein BJ, Igata M, Araki E (2006) AMPK and cell proliferation—AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol 574(Pt 1):63–71
Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K et al (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8(11):1288–1295
Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, Barnes JL, Abboud HE (2010) AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem 285(48):37503–37512
Lee JH, Kim JH, Kim JS, Chang JW, Kim SB, Park JS, Lee SK (2013) AMP-activated protein kinase inhibits TGF-beta-, angiotensin II-, aldosterone-, high glucose-, and albumin-induced epithelial-mesenchymal transition. Am J Physiol Renal Physiol 304(6):F686–F697
Luo X, Deng L, Lamsal LP, Xu W, Xiang C, Cheng L (2015) AMP-activated protein kinase alleviates extracellular matrix accumulation in high glucose-induced renal fibroblasts through mTOR signaling pathway. Cell Physiol Biochem 35(1):191–200
Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, Andreyev A, Quach T, Ly S, Shekhtman G et al (2013) AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest 123(11):4888–4899
Papadimitriou A, Peixoto EB, Silva KC, Lopes de Faria JM, Lopes de Faria JB (2014) Increase in AMPK brought about by cocoa is renoprotective in experimental diabetes mellitus by reducing NOX4/TGFbeta-1 signaling. J Nutr Biochem 25(7):773–784
Sharma S, Anjaneyulu M, Kulkarni SK, Chopra K (2006) Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology 76(2):69–75
Palsamy P, Subramanian S (2011) Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim Biophys Acta 1812(7):719–731
Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM et al (2010) Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab 11(6):554–565
Kim MY, Lim JH, Youn HH, Hong YA, Yang KS, Park HS, Chung S, Ko SH, Shin SJ, Choi BS et al (2013) Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1alpha axis in db/db mice. Diabetologia 56(1):204–217
Guan X, Nie L, He T, Yang K, Xiao T, Wang S, Huang Y, Zhang J, Wang J, Sharma K et al (2014) Klotho suppresses renal tubulo-interstitial fibrosis by controlling basic fibroblast growth factor-2 signalling. J Pathol 234(4):560–572
Wang SN, LaPage J, Hirschberg R (2000) Role of glomerular ultrafiltration of growth factors in progressive interstitial fibrosis in diabetic nephropathy. Kidney Int 57(3):1002–1014
Picard N, Baum O, Vogetseder A, Kaissling B, Le Hir M (2008) Origin of renal myofibroblasts in the model of unilateral ureter obstruction in the rat. Histochem Cell Biol 130(1):141–155
Faulkner JL, Szcykalski LM, Springer F, Barnes JL (2005) Origin of interstitial fibroblasts in an accelerated model of angiotensin II-induced renal fibrosis. Am J Pathol 167(5):1193–1205
Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H et al (2012) Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab 15(1):110–121
Yuan P, Xue H, Zhou L, Qu L, Li C, Wang Z, Ni J, Yu C, Yao T, Huang Y et al (2011) Rescue of mesangial cells from high glucose-induced over-proliferation and extracellular matrix secretion by hydrogen sulfide. Nephrol Dial Transplant 26(7):2119–2126
Lee YJ, Han HJ (2010) Troglitazone ameliorates high glucose-induced EMT and dysfunction of SGLTs through PI3K/Akt, GSK-3beta, Snail1, and beta-catenin in renal proximal tubule cells. Am J Physiol Renal Physiol 298(5):F1263–F1275
Liu Y (2011) Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7(12):684–696
Kobayashi M, Sugiyama H, Wang DH, Toda N, Maeshima Y, Yamasaki Y, Masuoka N, Yamada M, Kira S, Makino H (2005) Catalase deficiency renders remnant kidneys more susceptible to oxidant tissue injury and renal fibrosis in mice. Kidney Int 68(3):1018–1031
Sachse A, Wolf G (2007) Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol 18(9):2439–2446
Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ (2009) NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15(9):1077–1081
Ha H, Lee HB (2005) Reactive oxygen species amplify glucose signalling in renal cells cultured under high glucose and in diabetic kidney. Nephrology (Carlton) 10 Suppl:S7–S10
Susztak K, Raff AC, Schiffer M, Bottinger EP (2006) Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55(1):225–233
Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, Wilcox CS (2005) Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int 67(5):1890–1898
Spanier G, Xu H, Xia N, Tobias S, Deng S, Wojnowski L, Forstermann U, Li H (2009) Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J Physiol Pharmacol 60(Suppl 4):111–116
Sedeek M, Nasrallah R, Touyz RM, Hebert RL (2013) NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J Am Soc Nephrol 24(10):1512–1518
Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM et al (2010) Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299(6):F1348–F1358
Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP (2004) Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem 279(44):45935–45941
Sareila O, Kelkka T, Pizzolla A, Hultqvist M, Holmdahl R (2011) NOX2 complex-derived ROS as immune regulators. Antioxid Redox Signal 15:2197–2208
Liang J, Tian S, Han J, Xiong P (2014) Resveratrol as a therapeutic agent for renal fibrosis induced by unilateral ureteral obstruction. Ren Fail 36(2):285–291
Kitada M, Kume S, Imaizumi N, Koya D (2011) Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes 60(2):634–643
Ding DF, You N, Wu XM, Xu JR, Hu AP, Ye XL, Zhu Q, Jiang XQ, Miao H, Liu C et al (2010) Resveratrol attenuates renal hypertrophy in early-stage diabetes by activating AMPK. Am J Nephrol 31(4):363–374
McCarty MF, Barroso-Aranda J, Contreras F (2009) AMP-activated kinase may suppress NADPH oxidase activation in vascular tissues. Med Hypotheses 72(4):468–470
Schuhmacher S, Foretz M, Knorr M, Jansen T, Hortmann M, Wenzel P, Oelze M, Kleschyov AL, Daiber A, Keaney JF et al (2011) alpha1AMP-activated protein kinase preserves endothelial function during chronic angiotensin II treatment by limiting Nox2 upregulation. Arterioscler Thromb Vasc Biol 31(3):560–566
Acknowledgments
This study was supported by research grants from the National Natural Science Foundation of China (nos. 81270290, 81500561, and 81500567) and the project for overseas student from Ministry of Human Resources and Social Security of the People’s Republic of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests related to this study.
Additional information
Ting He and Jiachuan Xiong contributed equally to this work.
Rights and permissions
About this article
Cite this article
He, T., Xiong, J., Nie, L. et al. Resveratrol inhibits renal interstitial fibrosis in diabetic nephropathy by regulating AMPK/NOX4/ROS pathway. J Mol Med 94, 1359–1371 (2016). https://doi.org/10.1007/s00109-016-1451-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-016-1451-y