Abstract
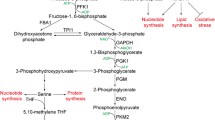
Nearly a hundred years of scientific research has revealed a notable preference of cancer cells to utilize aerobic glycolysis rather than mitochondrial oxidative phosphorylation for glucose-dependent ATP production, which is thought to be the root of tumor formation and growth. Glycolysis is a complex biochemical process that is mediated by multiple glycolytic genes. Besides regulating glucose metabolism, these genes are also suggested to possess various other functions related to cancer, including roles in cancer development and promotion, inhibition of apoptosis, cell cycle progression, and tumor metastasis. This article highlights the biological functions of glycolytic genes beyond their role in regulation of glycolysis and discusses their clinical implications, especially in regard to the use of glycolytic genes as biomarkers for early detection of cancer or as targets for novel anticancer treatments.

Similar content being viewed by others
References
Koppenol WH, Bounds PL, Dang CV (2011) Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer 11:325–337
Asano T, Shibasaki Y, Lin JL, Tsukuda K, Katagiri H, Ishihara H, Yazaki Y, Oka Y (1991) Expression of the GLUT1 glucose transporter increases thymidine uptake in Chinese hamster ovary cells at low glucose concentrations. Cancer Res 51:4450–4454
Ak I, Stokkel MP, Pauwels EK (2000) Positron emission tomography with 2-[18F]fluoro-2-deoxy-D-glucose in oncology. Part II. The clinical value in detecting and staging primary tumours. J Cancer Res Clin Oncol 126:560–574
Verrax J, Pedrosa RC, Beck R, Dejeans N, Taper H, Calderon PB (2009) In situ modulation of oxidative stress: a novel and efficient strategy to kill cancer cells. Curr Med Chem 16:1821–1830
Pastorino JG, Shulga N, Hoek JB (2002) Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem 277:7610–7618
Cardaci S, Desideri E, Ciriolo MR (2012) Targeting aerobic glycolysis: 3-bromopyruvate as a promising anticancer drug. J Bioenerg Biomembr 44:17–29
Pulselli R, Amadio L, Fanciulli M, Floridi A (1996) Effect of lonidamine on the mitochondrial potential in situ in Ehrlich ascites tumor cells. Anticancer Res 16:419–423
Funasaka T, Hogan V, Raz A (2009) Phosphoglucose isomerase/autocrine motility factor mediates epithelial and mesenchymal phenotype conversions in breast cancer. Cancer Res 69:5349–5356
Tanaka N, Haga A, Naba N, Shiraiwa K, Kusakabe Y, Hashimoto K, Funasaka T, Nagase H, Raz A, Nakamura KT (2006) Crystal structures of mouse autocrine motility factor in complex with carbohydrate phosphate inhibitors provide insight into structure-activity relationship of the inhibitors. J Mol Biol 356:312–324
Kim JW, Dang CV (2005) Multifaceted roles of glycolytic enzymes. Trends Biochem Sci 30:142–150
Ahmad A, Aboukameel A, Kong D, Wang Z, Sethi S, Chen W, Sarkar FH, Raz A (2011) Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells. Cancer Res 71:3400–3409
Lui VW, Wong EY, Ho K, Ng PK, Lau CP, Tsui SK, Tsang CM, Tsao SW, Cheng SH, Ng MH et al (2011) Inhibition of c-Met downregulates TIGAR expression and reduces NADPH production leading to cell death. Oncogene 30:1127–1134
Telang S, Clem BF, Klarer AC, Clem AL, Trent JO, Bucala R, Chesney J (2012) Small molecule inhibition of 6-phosphofructo-2-kinase suppresses t cell activation. J Transl Med 10:95
Rangarajan ES, Park H, Fortin E, Sygusch J, Izard T (2010) Mechanism of aldolase control of sorting nexin 9 function in endocytosis. J Biol Chem 285:11983–11990
Ritterson Lew C, Tolan DR (2012) Targeting of several glycolytic enzymes using rnai reveals aldolase affects cancer-cell proliferation through a non-glycolytic mechanism. J Biol Chem
Wang X, Lu Y, Yang J, Shi Y, Lan M, Liu Z, Zhai H, Fan D (2008) Identification of triosephosphate isomerase as an anti-drug resistance agent in human gastric cancer cells using functional proteomic analysis. J Cancer Res Clin Oncol 134:995–1003
Colell A, Green DR, Ricci JE (2009) Novel roles for GAPDH in cell death and carcinogenesis. Cell Death Differ 16:1573–1581
Sanchez-Arago M, Cuezva JM (2011) The bioenergetic signature of isogenic colon cancer cells predicts the cell death response to treatment with 3-bromopyruvate, iodoacetate or 5-fluorouracil. J Transl Med 9:19
Dai RP, Yu FX, Goh SR, Chng HW, Tan YL, Fu JL, Zheng L, Luo Y (2008) Histone 2B (H2B) expression is confined to a proper NAD+/NADH redox status. J Biol Chem 283:26894–26901
Xing C, LaPorte JR, Barbay JK, Myers AG (2004) Identification of GAPDH as a protein target of the saframycin antiproliferative agents. Proc Natl Acad Sci U S A 101:5862–5866
Hara MR, Cascio MB, Sawa A (2006) GAPDH as a sensor of NO stress. Biochim Biophys Acta 1762:502–509
Popanda O, Fox G, Thielmann HW (1998) Modulation of DNA polymerases alpha, delta and epsilon by lactate dehydrogenase and 3-phosphoglycerate kinase. Biochim Biophys Acta 1397:102–117
Myre MA, O'Day DH (2004) Calmodulin binds to and inhibits the activity of phosphoglycerate kinase. Biochim Biophys Acta 1693:177–183
Lay AJ, Jiang XM, Kisker O, Flynn E, Underwood A, Condron R, Hogg PJ (2000) Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature 408:869–873
Wang J, Ying G, Jung Y, Lu J, Zhu J, Pienta KJ, Taichman RS (2010) Characterization of phosphoglycerate kinase-1 expression of stromal cells derived from tumor microenvironment in prostate cancer progression. Cancer Res 70:471–480
Cheung EC, Vousden KH (2010) The role of p53 in glucose metabolism. Curr Opin Cell Biol 22:186–191
Evans MJ, Saghatelian A, Sorensen EJ, Cravatt BF (2005) Target discovery in small-molecule cell-based screens by in situ proteome reactivity profiling. Nat Biotechnol 23:1303–1307
Korse CM, Taal BG, Vincent A, van Velthuysen ML, Baas P, Buning-Kager JC, Linders TC, Bonfrer JM (2012) Choice of tumour markers in patients with neuroendocrine tumours is dependent on the histological grade. A marker study of Chromogranin A, neuron specific enolase, progastrin-releasing peptide and cytokeratin fragments. Eur J Cancer 48:662–671
Wang L, Liu P, Chen X, Geng Q, Lu Y (2012) Serum neuron-specific enolase is correlated with clinical outcome of patients with non-germinal center B cell-like subtype of diffuse large B-cell lymphoma treated with rituximab-based immunochemotherapy. Med Oncol 29:2153–2158
Tseng YT, Hsia JY, Chen CY, Lin NT, Chong PC, Yang CY (2011) Expression of the sperm fibrous sheath protein CABYR in human cancers and identification of alpha-enolase as an interacting partner of CABYR-a. Oncol Rep 25:1169–1175
Redlitz A, Fowler BJ, Plow EF, Miles LA (1995) The role of an enolase-related molecule in plasminogen binding to cells. Eur J Biochem 227:407–415
Wygrecka M, Marsh LM, Morty RE, Henneke I, Guenther A, Lohmeyer J, Markart P, Preissner KT (2009) Enolase-1 promotes plasminogen-mediated recruitment of monocytes to the acutely inflamed lung. Blood 113:5588–5598
Perconti G, Ferro A, Amato F, Rubino P, Randazzo D, Wolff T, Feo S, Giallongo A (2007) The kelch protein NS1-BP interacts with alpha-enolase/MBP-1 and is involved in c-Myc gene transcriptional control. Biochim Biophys Acta 1773:1774–1785
Soh M, Dunlevy JR, Garrett SH, Allen C, Sens DA, Zhou XD, Sens MA, Somji S (2012) Increased neuron specific enolase expression by urothelial cells exposed to or malignantly transformed by exposure to Cd(2)(+) or As(3)(+). Toxicol Lett 212:66–74
Tamada M, Suematsu M, Saya H (2012) Pyruvate kinase m2: multiple faces for conferring benefits on cancer cells. Clin Cancer Res 18:5554–5561
Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, Gao X, Aldape K, Lu Z (2011) Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature 480:118–122
Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, Aldape K, Hunter T, Alfred Yung WK, Lu Z (2012) PKM2 Phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell 150:685–696
Grosse F, Nasheuer HP, Scholtissek S, Schomburg U (1986) Lactate dehydrogenase and glyceraldehyde-phosphate dehydrogenase are single-stranded DNA-binding proteins that affect the DNA-polymerase-alpha-primase complex. Eur J Biochem 160:459–467
Zheng L, Roeder RG, Luo Y (2003) S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell 114:255–266
Sasaki H, Shitara M, Yokota K, Hikosaka Y, Moriyama S, Yano M, Fujii Y (2012) Overexpression of GLUT1 correlates with Kras mutations in lung carcinomas. Mol Med Rep 5:599–602
Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, Xu Y, Wonsey D, Lee LA, Dang CV (2000) Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem 275:21797–21800
Mobasheri A, Richardson S, Mobasheri R, Shakibaei M, Hoyland JA (2005) Hypoxia inducible factor-1 and facilitative glucose transporters GLUT1 and GLUT3: putative molecular components of the oxygen and glucose sensing apparatus in articular chondrocytes. Histol Histopathol 20:1327–1338
Amann T, Maegdefrau U, Hartmann A, Agaimy A, Marienhagen J, Weiss TS, Stoeltzing O, Warnecke C, Scholmerich J, Oefner PJ et al (2009) GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol 174:1544–1552
Casneuf VF, Fonteyne P, Van Damme N, Demetter P, Pauwels P, de Hemptinne B, De Vos M, Van de Wiele C, Peeters M (2008) Expression of SGLT1, Bcl-2 and p53 in primary pancreatic cancer related to survival. Cancer Investig 26:852–859
Lai B, Xiao Y, Pu H, Cao Q, Jing H, Liu X (2012) Overexpression of SGLT1 is correlated with tumor development and poor prognosis of ovarian carcinoma. Arch Gynecol Obstet 285:1455–1461
Guo GF, Cai YC, Zhang B, Xu RH, Qiu HJ, Xia LP, Jiang WQ, Hu PL, Chen XX, Zhou FF et al (2011) Overexpression of SGLT1 and EGFR in colorectal cancer showing a correlation with the prognosis. Med Oncol 28(Suppl 1):S197–S203
Dang CV (2007) The interplay between MYC and HIF in the Warburg effect. Ernst Schering Found Symp Proc 35–53
Pedersen PL, Mathupala S, Rempel A, Geschwind JF, Ko YH (2002) Mitochondrial bound type II hexokinase: a key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention. Biochim Biophys Acta 1555:14–20
Bando H, Atsumi T, Nishio T, Niwa H, Mishima S, Shimizu C, Yoshioka N, Bucala R, Koike T (2005) Phosphorylation of the 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase/PFKFB3 family of glycolytic regulators in human cancer. Clin Cancer Res 11:5784–5792
Mendoza EE, Pocceschi MG, Kong X, Leeper DB, Caro J, Limesand KH, Burd R (2012) Control of glycolytic flux by AMP-activated protein kinase in tumor cells adapted to low pH. Transl Oncol 5:208–216
Peng SY, Lai PL, Pan HW, Hsiao LP, Hsu HC (2008) Aberrant expression of the glycolytic enzymes aldolase B and type II hexokinase in hepatocellular carcinoma are predictive markers for advanced stage, early recurrence and poor prognosis. Oncol Rep 19:1045–1053
Mikuriya K, Kuramitsu Y, Ryozawa S, Fujimoto M, Mori S, Oka M, Hamano K, Okita K, Sakaida I, Nakamura K (2007) Expression of glycolytic enzymes is increased in pancreatic cancerous tissues as evidenced by proteomic profiling by two-dimensional electrophoresis and liquid chromatography-mass spectrometry/mass spectrometry. Int J Oncol 30:849–855
Zhang XZ, Xiao ZF, Li C, Xiao ZQ, Yang F, Li DJ, Li MY, Li F, Chen ZC (2009) Triosephosphate isomerase and peroxiredoxin 6, two novel serum markers for human lung squamous cell carcinoma. Cancer Sci 100:2396–2401
Higashimura Y, Nakajima Y, Yamaji R, Harada N, Shibasaki F, Nakano Y, Inui H (2011) Up-regulation of glyceraldehyde-3-phosphate dehydrogenase gene expression by HIF-1 activity depending on Sp1 in hypoxic breast cancer cells. Arch Biochem Biophys 509:1–8
Tang Z, Yuan S, Hu Y, Zhang H, Wu W, Zeng Z, Yang J, Yun J, Xu R, Huang P (2012) Over-expression of GAPDH in human colorectal carcinoma as a preferred target of 3-bromopyruvate propyl ester. J Bioenerg Biomembr 44:117–125
He H, Lee MC, Zheng LL, Zheng L, Luo Y (2013) Integration of the metabolic/redox state, histone gene switching, DNA replication and S-phase progression by moonlighting metabolic enzymes. Biosci Rep 33:e00018
Hwang TL, Liang Y, Chien KY, Yu JS (2006) Overexpression and elevated serum levels of phosphoglycerate kinase 1 in pancreatic ductal adenocarcinoma. Proteomics 6:2259–2272
Zieker D, Konigsrainer I, Weinreich J, Beckert S, Glatzle J, Nieselt K, Buhler S, Loffler M, Gaedcke J, Northoff H et al (2010) Phosphoglycerate kinase 1 promoting tumor progression and metastasis in gastric cancer—detected in a tumor mouse model using positron emission tomography/magnetic resonance imaging. Cell Physiol Biochem 26:147–154
Ren F, Wu H, Lei Y, Zhang H, Liu R, Zhao Y, Chen X, Zeng D, Tong A, Chen L et al (2010) Quantitative proteomics identification of phosphoglycerate mutase 1 as a novel therapeutic target in hepatocellular carcinoma. Mol Cancer 9:81
Huang LJ, Chen SX, Luo WJ, Jiang HH, Zhang PF, Yi H (2006) Proteomic analysis of secreted proteins of non-small cell lung cancer. Ai Zheng 25:1361–1367
Capello M, Ferri-Borgogno S, Cappello P, Novelli F (2011) alpha-Enolase: a promising therapeutic and diagnostic tumor target. FEBS J 278:1064–1074
Bayley JP, Devilee P (2011) The Warburg effect in 2012. Curr Opin Oncol 2012(24):62–67
Tennant DA: PK-M2 makes cells sweeter on HIF1. Cell 145:647–649
Bluemlein K, Gruning NM, Feichtinger RG, Lehrach H, Kofler B, Ralser M (2011) No evidence for a shift in pyruvate kinase PKM1 to PKM2 expression during tumorigenesis. Oncotarget 2:393–400
Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW et al (2013) PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell 155:397–409
Fantin VR, St-Pierre J, Leder P (2006) Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9:425–434
Yalcin A, Telang S, Clem B, Chesney J (2009) Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol 86:174–179
Kondoh H, Lleonart ME, Gil J, Wang J, Degan P, Peters G, Martinez D, Carnero A, Beach D (2005) Glycolytic enzymes can modulate cellular life span. Cancer Res 65:177–185
Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, Hawkins C, Guha A (2011) Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med 208:313–326
Xu W, Seiter K, Feldman E, Ahmed T, Chiao JW (1996) The differentiation and maturation mediator for human myeloid leukemia cells shares homology with neuroleukin or phosphoglucose isomerase. Blood 87:4502–4506
Weihua Z, Tsan R, Huang WC, Wu Q, Chiu CH, Fidler IJ, Hung MC (2008) Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell 13:385–393
Attner B, Landin-Olsson M, Lithman T, Noreen D, Olsson H (2012) Cancer among patients with diabetes, obesity and abnormal blood lipids: a population-based register study in Sweden. Cancer Causes Control 23:769–777
Taubes G (2012) Cancer research. Cancer prevention with a diabetes pill? Science 335:29
Mor I, Cheung EC, Vousden KH (2011) Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb Symp Quant Biol 76:211–216
Green DR, Chipuk JE (2006) p53 and metabolism: inside the TIGAR. Cell 126:30–32
Ros S, Santos CR, Moco S, Baenke F, Kelly G, Howell M, Zamboni N, Schulze A (2012) Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer Discov 2:328–343
Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM et al (2010) Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science 329:1492–1499
Scatena R, Bottoni P, Pontoglio A, Mastrototaro L, Giardina B (2008) Glycolytic enzyme inhibitors in cancer treatment. Expert Opin Investig Drugs 17:1533–1545
Orosz F, Olah J, Ovadi J (2009) Triosephosphate isomerase deficiency: new insights into an enigmatic disease. Biochim Biophys Acta 1792:1168–1174
McHarg J, Littlechild JA (1996) Studies with inhibitors of the glycolytic enzyme phosphoglycerate kinase for potential treatment of cardiovascular and respiratory disorders. J Pharm Pharmacol 48:201–205
Krishnan P, Gullen EA, Lam W, Dutschman GE, Grill SP, Cheng YC (2003) Novel role of 3-phosphoglycerate kinase, a glycolytic enzyme, in the activation of L-nucleoside analogs, a new class of anticancer and antiviral agents. J Biol Chem 278:36726–36732
Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A, Rasku MA, Arumugam S, Dean WL, Eaton J et al (2008) Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther 7:110–120
Tomaino B, Cappello P, Capello M, Fredolini C, Sperduti I, Migliorini P, Salacone P, Novarino A, Giacobino A, Ciuffreda L et al (2011) Circulating autoantibodies to phosphorylated alpha-enolase are a hallmark of pancreatic cancer. J Proteome Res 10:105–112
Choi SR, Pradhan A, Hammond NL, Chittiboyina AG, Tekwani BL, Avery MA (2007) Design, synthesis, and biological evaluation of Plasmodium falciparum lactate dehydrogenase inhibitors. J Med Chem 50:3841–3850
Martinez-Outschoorn UE, Pestell RG, Howell A, Tykocinski ML, Nagajyothi F, Machado FS, Tanowitz HB, Sotgia F, Lisanti MP (2011) Energy transfer in “parasitic” cancer metabolism: mitochondria are the powerhouse and Achilles’ heel of tumor cells. Cell Cycle 10:4208–4216
Caro P, Kishan AU, Norberg E, Stanley IA, Chapuy B, Ficarro SB, Polak K, Tondera D, Gounarides J, Yin H et al (2012) Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell 22:547–560
Pollak M (2013) Targeting oxidative phosphorylation: why, when, and how. Cancer Cell 23:263–264
Wang PY, Ma W, Park JY, Celi FS, Arena R, Choi JW, Ali QA, Tripodi DJ, Zhuang J, Lago CU et al (2013) Increased oxidative metabolism in the Li-Fraumeni syndrome. N Engl J Med 368:1027–1032
Acknowledgments
This review was supported by the National Basic Research Program of China, No. 2011CB504305; National Natural Science Foundation of China (NSFC), No. 30930101 and 81161120410; and China Postdoctoral Science Foundation funded project, No. 2011M501300.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, ZY., Xiao, L., Bode, A.M. et al. Glycolytic genes in cancer cells are more than glucose metabolic regulators. J Mol Med 92, 837–845 (2014). https://doi.org/10.1007/s00109-014-1174-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-014-1174-x