Abstract
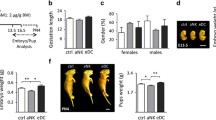
Implantation of mammalian embryos into their mother’s uterus ensures optimal nourishment and protection throughout development. Complex molecular interactions characterize the implantation process, and an optimal synchronization of the components of this embryo-maternal dialogue is crucial for a successful reproductive outcome. In the present study, we investigated the role of dendritic cells (DC) during implantation process using a transgenic mouse system (DTRtg) that allows transient depletion of CD11c+ cells in vivo through administration of diphtheria toxin. We observed that DC depletion impairs the implantation process, resulting in a reduced breeding efficiency. Furthermore, the maturity of uterine natural killer cells at dendritic cell knockout (DCKO) implantation sites was affected as well; as demonstrated by decreased perforin expression and reduced numbers of periodic-acid-Schiff (PAS)-positive cells. This was accompanied by disarrangements in decidual vascular development. In the present study, we were also able to identify a novel DC-dependent protein, phosphatidylinositol transfer protein β (PITPβ), involved in implantation and trophoblast development using a proteomic approach. Indeed, DCKO mice exhibited substantial anomalies in placental development, including hypocellularity of the spongiotrophoblast and labyrinthine layers and reduced numbers of trophoblast giant cells. Giant cells also down-regulated their expression of two characteristic markers of trophoblast differentiation, placental lactogen 1 and proliferin. In view of these findings, dendritic cells emerge as possible modulators in the orchestration of events leading to the establishment and maintenance of pregnancy.





Similar content being viewed by others
References
Aluvihare VR, Kallikourdis M, Betz AG (2005) Tolerance, suppression and the fetal allograft. J Mol Med 83.2:88–96 (Feb)
Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K (2000) Embryo implantation. Dev Biol 223.2:217–237 (Jul 15)
Norwitz ER, Schust DJ, Fisher SJ (2001) Implantation and the survival of early pregnancy. N Engl J Med 345.13:1400–1408 (Nov 8)
Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr, Shyamala G, Conneely OM, O'Malley BW (1995) Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9.18:2266–2278 (Sep 15)
Moffett-King A (2002) Natural killer cells and pregnancy. Nat Rev Immunol 2.9:656–663 (Sep)
Croy BA, Esadeg S, Chantakru S, van den Heuvel M, Paffaro VA, He H, Black GP, Ashkar AA, Kiso Y, Zhang J (2003) Update on pathways regulating the activation of uterine natural killer cells, their interactions with decidual spiral arteries and homing of their precursors to the uterus. J Reprod Immunol 59.2:175–191 (Aug)
Verma S, Hiby SE, Loke YW, King A (2000) Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod 62.4:959–968 (Apr)
Shinozaki M, Hirahashi J, Lebedeva T, Liew FY, Salant DJ, Maron R, Kelley VR (2002) IL-15, a survival factor for kidney epithelial cells, counteracts apoptosis and inflammation during nephritis. J Clin Invest 109.7:951–960 (Apr)
Waldmann TA, Dubois S, Tagaya Y (2001) Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity 14.2:105–110 (Feb)
Ain R, Canham LN, Soares MJ (2003) Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol 260.1:176–190 (Aug 1)
Ashkar AA, Di Santo JP, Croy BA (2000) Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med 192.2:259–270 (Jul 17)
Croy BA, He H, Esadeg S, Wei Q, McCartney D, Zhang J, Borzychowski A, Ashkar AA, Black GP, Evans SS, Chantakru S, van den Heuvel M, Paffaro VA Jr, Yamada AT (2003) Uterine natural killer cells: insights into their cellular and molecular biology from mouse modelling. Reproduction 126.2:149–160 (Aug)
Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, Croy BA (1997) Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod 56.1:169–179 (Jan)
Blois SM, Alba Soto CD, Tometten M, Klapp BF, Margni RA, Arck PC (2004) Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol Reprod 70.4:1018–1023 (Apr)
Haimovici F, Anderson DJ (1993) Cytokines and growth factors in implantation. Microsc Res Tech 25.3:201–207 (Jun 15)
Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres-Bartho J, Rabinovich GA, Arck PC (2007) A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med 13(12):1450–1457 (Dec)
Laskarin G, Kämmerer U, Rukavina D, Thomson AW, Fernandez N, Blois SM (2007) Antigen-presenting cells and materno-fetal tolerance: an emerging role for dendritic cells. Am J Reprod Immunol 58(3):255–67 (Sep)
Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, Bougras G, Muller WA, Moretta L, Munz C (2004) Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A 101.47:16606–16611 (Nov 23)
Yu Y, Hagihara M, Ando K, Gansuvd B, Matsuzawa H, Tsuchiya T, Ueda Y, Inoue H, Hotta T, Kato S (2001) Enhancement of human cord blood CD34+ cell derived NK cell cytotoxicity by dendritic cells. J Immunol 166:1590–1600
Piccioli D, Sbrana S, Melandri E, Valiante N (2002) Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med 195:335–341
Blois SM, Barrientos G, Garcia MG, Orsal AS, Tometten M, Cordo-Russo R, Klapp BF, Santoni A, Fernandez N, Terness P, Arck PC (2008) Interaction between dendritic cells and natural killer cells during pregnancy in mice. J Mol Med (doi:10.1007/s00109-008-0342-2)
Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA (2002) In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 17.2:211–220 (Aug)
Probst HC, Tschannen K, Odermatt B, Schwendener R, Zinkernagel RM, Van Den Broek M (2005) Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin Exp Immunol 141.3:398–404 (Sep)
Kammerer U, Eggert AO, Kapp M, McLellan AD, Geijtenbeek TB, Dietl J, van Kooyk Y, Kampgen E (2003) Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am J Pathol 162.3:887–896 (Mar)
Ye W, Zheng LM, Young JD, Liu CC (1996) The involvement of interleukin (IL)-15 in regulating the differentiation of granulated metrial gland cells in mouse pregnant uterus. J Exp Med 184.6:2405–2410 (Dec 1)
Wang H, Dey S (2006) Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 7.3:185–199
Yang Z, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings B (2003) Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem 278:32124–32131
Riley J, Moley K (2006) Glucose utilization and the PI3-K-pathway: mechanisms for cell survival in preimplantation embryos. Reproduction 131:823–835
Qiu Q, Yang M, Tsang B, Gruslin A (2004) EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction 128:355–363
Kamei T, Jones S, Chapman B, McGonigle K, Dai G, Soares M (2002) The phosphatidylinositol 3-Kinase/Akt signaling pathway modulates the endocrine differentiation of trophoblast cells. Mol Endocrinol 16(7):1469–1481
Faria TN, Soares MJ (1991) Trophoblast cell differentiation: establishment, characterization, and modulation of a rat trophoblast cell line expressing members of the placental prolactin family. Endocrinology 129.6:2895–2906 (Dec)
Blois SM, Kammerer U, Soto CA, Tometten MC, Shaikly V, Barrientos G, Jurd R, Rukavina D, Thomson AW, Klapp BF, Fernandez N, Arck PC (2007) Dendritic cells: key to fetal tolerance? Biol Reprod 77.4:590–598 (Oct)
Parr MB, Parr EL (1989) Immunohistochemical investigation of secretory component and immunoglobulin A in the genital tract of the female rat. J Reprod Fertil 85.1:105–113 (Jan)
Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, Croy BA (2003) Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol 171.6:2937–2944 (Sep 15)
Barber EM, Pollard JW (2003) The uterine NK cell population requires IL-15 but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J Immunol 171.1:37–46 (Jul 1)
Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A (2007) Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 26.4:503–517 (Apr)
Burnett TG, Hunt JS (2000) Nitric oxide synthase-2 and expression of perforin in uterine NK cells. J Immunol 164.10:5245–5250 (May 15)
Croy BA, Luross JA, Guimond MJ, Hunt JS (1996) Uterine natural killer cells: insights into lineage relationships and functions from studies of pregnancies in mutant and transgenic mice. Nat Immunol 15.1:22–33
Croy BA, Ashkar AA, Foster RA, DiSanto JP, Magram J, Carson D, Gendler SJ, Grusby MJ, Wagner N, Muller W, Guimond MJ (1997) Histological studies of gene-ablated mice support important functional roles for natural killer cells in the uterus during pregnancy. J Reprod Immunol 35.2:111–133 (Nov 15)
Wang C, Tanaka T, Nakamura H, Umesaki N, Hirai K, Ishiko O, Ogita S, Kaneda K (2003) Granulated metrial gland cells in the murine uterus: localization, kinetics, and the functional role in angiogenesis during pregnancy. Microsc Res Tech 60.4:420–429 (Mar 1)
Croy BA, Ashkar AA, Minhas K, Greenwood JD (2000) Can murine uterine natural killer cells give insights into the pathogenesis of preeclampsia? J Soc Gynecol Investig 7.1:12–20 (Jan–Feb)
Cockcroft S, Carvou N (2007) Biochemical and biological functions of class I phosphatidylinositol transfer proteins. Biochim Biophys Acta 1771.6:677–691 (Jun)
Lee KF, Kwok KL, Chung MK, Lee YL, Chow JF, Yeung WS (2005) Phospholipid transfer protein (PLTP) mRNA expression is stimulated by developing embryos in the oviduct. J Cell Biochem 95.4:740–749 (Jul 1)
Cunningham E, Thomas G, Ball A, Hiles I, Cockcroft S (1995) Phosphatidylinositol transfer protein dictates the rate of inositol trisphosphate production by promoting the synthesis of PIP2. Curr Biol 5.7:775–783
Martin T (1995) Intracellular signalling. New directions for phosphatidylinositol transfer. Curr Biol 5.9:990–992
Panaretou C, Domin J, Cockcroft S, Waterfield M (1997) Characterization of p150, an adaptor protein for the human phosphatidylinositol (PtdIns) 3-kinase. Substrate presentation by phosphatidylinositol transfer protein to the p150.Ptdins 3-kinase complex. J Biol Chem 272.4:2477–2485
Acknowledgments
This work was supported by research grants from the Charité (AF-2007-011) to S.M.B. G.B. and R.C-R. received a scholarship from the German Student Exchange Program (Deutscher Akademischer Austauschdienst). S.M.B is a fellow of the Habilitation program at the Charité, University Medicine Berlin. P.C.A. and S.M.B. are part of the EMBIC Network of Excellence, co-financed by the European Commission throughout the FP6 framework program Life Science, Genomics and Biotechnology for Health.
Author contributions
G.K. performed all the experiments and contributed to manuscript writing; V.S. designed and performed proteomics experiments. P.F., G.B., and P.M. performed histological analysis and animal experiments; R.C-R. and F.R. assisted with the PCR experiments; I.C. and M.M. assisted with MALDI-MS experiments; B.F.K and N.F provided advice, P.C.A. contributed with reagents and S.M.B. supervised the work, designed the experiments and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Pierre Frank and Valerie Shaikly contributed equally to this work.
Rights and permissions
About this article
Cite this article
Krey, G., Frank, P., Shaikly, V. et al. In vivo dendritic cell depletion reduces breeding efficiency, affecting implantation and early placental development in mice. J Mol Med 86, 999–1011 (2008). https://doi.org/10.1007/s00109-008-0379-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-008-0379-2