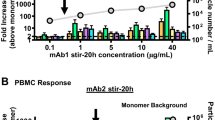
Abstract
Tumor-derived exosomes have been regarded as a new kind of cancer vaccine; however, their therapeutic efficacy needs to be further improved. Superantigen staphylococcal enterotoxin A (SEA)-coated tumor cells have been shown to potently induce tumor-specific T cell response. To increase efficacy of tumor-derived exosomes to induce antitumor immune response, we modified the exosomes by protein transfer of SEA tailed with a highly hydrophobic transmembrane domain (SEA-TM) and designated those SEA-TM-anchored exosomes as Exo/SEA-TM. We found the exosomes secreted from murine lymphoma E.G7-OVA cell line were round vesicles with the sizes of 40–100 nm limited by a bilayer membrane. Interestingly, the inner structure of the exosomes were visible under the transmission electron microscope; those “honeycomb-like” inner structure has not been described by other labs. Immunization with Exo/SEA-TM inhibited tumor growth and prolonged survival of the mice challenged with parental tumor cells more significantly than with exosomes (Exo) and even more than with the mixture of exosomes and SEA-TM. The results of mixed lymphocyte–tumor reaction (MLTR) showed that the increased IL-2, IFN-gamma secretion, and specific cytotoxic T lymphocyte (CTL) could be effectively induced from the splenic lymphocytes of the mice immunized with Exo/SEA-TM. In vivo depletion experiments showed that CD8+ T cells are the main effector cells, and both CD4+ T cells and NK cells are also involved in the antitumor effect of Exo/SEA-TM immunization. Therefore, tumor-derived exosomes surface anchored with SEA-TM can efficiently induce tumor-specific CTL thereby resulting in more potent inhibition of tumor growth. Our data provide an efficient and novel approach to tumor immunotherapy by protein modification of tumor-derived exosomes.






Similar content being viewed by others
Change history
15 January 2020
The corrected Fig. 1 image and caption is presented in this paper.
15 January 2020
The corrected Fig. 1 image and caption is presented in this paper.
Abbreviations
- Exo:
-
conventional exosomes
- Exo/SEA-TM:
-
SEA-TM-anchored exosomes
- HSC70:
-
heat shock cognate 70 protein
- SEA:
-
staphylococcal enterotoxin A
- TdR:
-
thymidine
- TEXs:
-
tumor-derived exosomes
- TM:
-
transmembrane
References
Taieb J, Chaput N, Zitvogel L (2005) Dendritic cell-derived exosomes as cell-free peptide-based vaccines. Crit Rev Immunol 25:215–223
Thery C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2:569–579
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic-cell-derived exosomes. Nat Med 4:594–600
Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK(2005) A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 3:9
Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L (2005) Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med 3:10
Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L (2001) Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 7:297–303
Chaput N, Schartz NE, Andre F, Taieb J, Novault S, Bonnaventure P, Aubert N, Bernard J, Lemonnier F, Merad M, Adema G, Adams M, Ferrantini M, Carpentier AF, Escudier B, Tursz T, Angevin E, Zitvogel L (2004) Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol 172:2137–2146
Soo Hoo W, Lundeen KA, Kohrumel JR, Pham NL, Brostoff SW, Bartholomew RM, Carlo DJ (1999) Tumor cell surface expression of granulocyte-macrophage colony-stimulating factor elicits antitumor immunity and protects from tumor challenge in the P815 mouse mastocytoma tumor model. J Immunol 162:7343–73439
Nagarajan S, Selvaraj P (2002) Glycolipid-anchored IL-12 expressed on tumor cell surface induces antitumor immune response. Cancer Res 62:2869–2874
McHugh RS, Nagarajan S, Wang Y, Sell KW, Selvaraj P (1999) Protein transfer of glycosyl-phosphatidylinositol-B7-1 into tumor cell membranes: a novel approach to tumor immunotherapy. Cancer Res 59:2433–2437
Wang Q, Yu H, Zhang L, Ju D, Pan J, Xia D, He L, Wang J, Cao X (2001) Vaccination with IL-18 gene-modified, superantigen-coated tumor cells elicits potent antitumor immune response. J Cancer Res Clin Oncol 27:718–726
Ma W, Yu H, Wang Q, Bao J, Yan J, Jin H (2004) In vitro biological activities of transmembrane superantigen staphylococcal enterotoxin A fusion protein. Cancer Immunol Immunother 53:118–124
Xiu F, Yang Y, Cai Z, Wang J, Cao X (2004) Isolation and characterization of exosomes derived from tumor cells genetically expressing model antigen. J Microbiol Immunol 2:278–285
Dai S, Wan T, Wang B, Zhou X, Xiu F, Chen T, Wu Y, Cao X (2005) More efficient induction of HLA-A*0201-restricted and CEA-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumour cells. Clin Cancer Res 11:7554–7563
Clayton A, Court J, Navabi H, Adams M, Mason MD, Hobot JA, Newman GR, Jasani B (2001) Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J Immunol Methods 247:163–174
Wu Y, Wan T, Zhou X, Wang B, Yang F, Li N, Chen G, Dai S, Liu S, Zhang M, Cao X (2005) HSP70-like protein 1 fusion protein enhances induction of carcinoembryonic antigen-specific CD8+ CTL response by dendritic cell vaccine. Cancer Res 65:4947–4954
Xia DJ, Zhang WP, Zheng S, Wang J, Pan JP, Wang Q, Zhang LH, Hamada H, Cao X (2002) Lymphotactin cotransfection enhances the therapeutic efficacy of dendritic cells genetically modified with melanoma antigen gp100. Gene Ther 9:592–601
Chen W, Wang J, hao C, Liu S, Yu Y, Wang Q, Cao X (2006). Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur J Immunol 36:1598–1607
Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ (1998) Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem 273:20121–20127
Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J, Slot JW, Geuze HJ, Stoorvogel W (2003) Proteomic and biochemical analyses of human B-cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem 278:10963–10972
Torres BA, Kominsky SL, Perrin GQ, Hobeika AC, Johnson HM (2001) Superantigens: the good, the bad, and the ugly. Exp Biol Med 226:164–176
Nagarajan S, Anderson M, Ahmed SN, Sell KW, Selvaraj P (1995) Purification and optimization of functional reconstitution on the surface of leukemic cell lines of GPI-anchored Fcg receptor III. J Immunol Methods 184:241–251
Fleischer B, Schrezenmeier H (1998) T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med 167:1697–1707
Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD Jr, Thomson AW (2004) Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104:3257–3266
Ou LS, Goleva E, Hall C, Leung DY (2004) T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J Allergy Clin Immunol 113:756–763
Udono H, Yamano T, Kawabata Y, Ueda M, Yui K (2001) Generation of cytotoxic T lymphocytes by MHC class I ligands fused to heat shock cognate protein 70. Int Immunol 13:1233–1242
Wan T, Zhou X, Chen G, An H, Chen T, Zhang W, Liu S, Jiang Y, Yang F, Wu Y, Cao X (2005) Novel heat shock protein HSP70L1 activates dendritic cells and acts as a Th1 polarizing adjuvant. Blood 103:1747–1754
Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G (2005) Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res 65:5238–5247
Acknowledgements
We thank Drs. Xiangyang Zhou, Minghui Zhang, Lihuang Zhang for their helpful discussion
This work was supported by grants from the National Natural Science Foundation of China (30490240, 30121002) and the National Key Basic Research Program of China (2001CB510002).
Fangming Xiu and Zhijian Cai contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiu, F., Cai, Z., Yang, Y. et al. Surface anchorage of superantigen SEA promotes induction of specific antitumor immune response by tumor-derived exosomes. J Mol Med 85, 511–521 (2007). https://doi.org/10.1007/s00109-006-0154-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-006-0154-1