Abstract
Introduction
Secondary abdominal compartment syndrome (ACS) can occur in trauma patients without abdominal injuries. Surgical management of patients presenting with secondary ACS after isolated traumatic lower extremity vascular injury (LEVI) continues to evolve, and associated outcomes remain unknown.
Methods
From January 2006 to September 2011, 191 adult trauma patients presented to the Ryder Trauma Center, an urban level I trauma center in Miami, Florida with traumatic LEVIs. Among them 10 (5.2 %) patients were diagnosed with secondary ACS. Variables collected included age, gender, mechanism of injury, and clinical status at presentation. Surgical data included vessel injury, technical aspects of repair, associated complications, and outcomes.
Results
Mean age was 37.4 ± 18.0 years (range 16–66 years), and the majority of patients were males (8 patients, 80 %). There were 7 (70 %) penetrating injuries (5 gunshot wounds and 2 stab wounds), and 3 blunt injuries with mean Injury Severity Score (ISS) 21.9 ± 14.3 (range 9–50). Surgical management of LEVIs included ligation (4 patients, 40 %), primary repair (1 patient, 10 %), reverse saphenous vein graft (2 patients, 20 %), and PTFE interposition grafting (3 patients, 30 %). The overall mortality rate in this series was 60 %.
Conclusions
The association between secondary ACS and lower extremity vascular injuries carries high morbidity and mortality rates. Further research efforts should focus at identifying parameters to accurately determine resuscitation goals, and therefore, prevent such a devastating condition.
Similar content being viewed by others
Introduction
Abdominal compartment syndrome (ACS) is a condition of serious multi-organ dysfunction resulting from sustained intra-abdominal hypertension (IAH), affecting the cardiovascular, respiratory, and renal systems [1]. IAH leading to ACS is a result of massive swelling of the intra-peritoneal viscera, which can occur with (primary) or without (secondary) intra-peritoneal injury [2, 3]. Secondary ACS (SACS) is a life-threatening condition in critically ill trauma patients, if not confronted promptly, resulting in multi-system organ failure and is associated with mortality rates up to 100 % in some series [4, 5]. SACS most often occurs after trauma (hemorrhagic shock), burn-related injury, or septic shock requiring massive resuscitation [5]. The incidence is estimated at 8 % of all shock trauma patients requiring aggressive resuscitation and 0.7 % of all trauma ICU admissions [6].
There has been a resurgent interest over the past several years in better understanding the pathogenesis and management of secondary ACS with the widespread acceptance of staged laparotomy in trauma [7, 8]. Most published series, SACS includes a wide range of etiologies, both trauma and non-trauma, therefore focused management and outcomes of patients with isolated traumatic lower extremity vascular injury (LEVI) remain unclear. We aim at describing our experience with SACS after traumatic LEVI, thereby focusing on initial presentation, surgical management, and outcomes.
Methods
Between January 2006 and September 2011, 191 adult trauma patients presented to the Ryder Trauma Center, an urban level I trauma center in Miami, Florida with traumatic LEVIs. Among them 10 (5.2 %) patients were diagnosed with SACS and were included in this analysis. Institutional review board approval for this study was obtained, waiving the need for informed consent. Patients with concurrent intra-abdominal injury were excluded from this study. Data was retrospectively collected by a single investigator and included patient’s demographics, mechanism of injury, type of vascular injury, and physiologic status on initial assessment. Laboratory and clinical data evaluation included emergency department (ED) base deficit, serum pH, and hematocrit levels. The amount of crystalloids and colloids given during the operative fluid resuscitation phase was recorded. Injury severity was determined based on the Revised Trauma Score (RTS), Injury Severity Score (ISS), and admission Glasgow Coma Scale (GCS). A severe injury was categorically defined as an ISS of greater than 15. Outcome variables included mortality, surgical procedures, complications, hospital length of stay, and intensive care unit (ICU) length of stay.
The diagnosis of SACS was established based on the clinical presentation, with evidence of physiologic compromise. Criteria included intra-abdominal pressure greater than 20 mmHg and progressive organ dysfunction (urinary output <0.5 mL/kg/h, PaO2/FiO2 ratio <200, peak airway pressure >45 cmH2O, or cardiac index <3 L/min m2) despite resuscitation.
The operative management of all patients was similar in this series. A midline celiotomy from the xiphisternum to the pubic symphysis was performed to establish adequate abdominal decompression at the time of diagnosis. The abdominal contents were explored to confirm there were no associated injuries. Upon completion a perforated sterile plastic sheet was placed in addition to closed suction drains and an Ioban™ dressing 60 cm × 45 cm (3M™ Health Care, St. Paul, MN) to establish a watertight seal. All patients were then transferred to the trauma intensive care unit (ICU) for continued postoperative care and resuscitation. All survivors returned back to the operating room within 48 to 72 h to attempt definitive closure.
Results
Of the 10 patients included in this series, there were 7 (70 %) penetrating injuries (5 gunshot wounds and 2 stab wounds) and 3 blunt injuries (2 pedestrians struck and 1 motor vehicle collision). The mean age was 37.4 ± 18.0 years (range 16–66 years), and the majority of patients were males (8 patients, 80 %). Demographics and clinical data are summarized in Table 1. The presence of hard signs was evaluated in patients who presented with a measurable blood pressure. All patients were immediately transported to the operating room for vascular repair. Ankle brachial index (ABI) and preoperative computed tomography were not performed in any patient upon admission. Mean ISS and RTS were 21.9 ± 14.3 (range 9–50) and 5.8 ± 4.8 (range 0–12), respectively. Five patients had no recorded blood pressure on arrival, and the remaining had an overall mean admission systolic blood pressure of 108.8 ± 52.1 mmHg (range 65–190 mmHg). The majority of patients had severe acidosis on initial blood gas, with a mean pH of 6.97 ± 0.37 (range 6.27–7.33). A base deficit was seen in all patients on initial presentation with mean of −17.1 ± 11.2 (range −37 to −2) (Table 1). The average amount of crystalloid used intraoperatively was 12.8 ± 8.2 L (range 3–30 L). The mean total units of packed red blood cells (PRBC), fresh frozen plasma (FFP), and platelets transfused were 25.6 ± 16.31 units (range 9–53), 13.5 ± 10.6 units (range 4–36), and 11.5 ± 9.4 units (range 0–30), respectively.
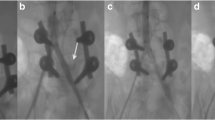
The patterns of vascular injuries are summarized in Table 1. Surgical management of LEVIs included ligation (4 patients, 40 %), primary repair (1 patient, 10 %), reverse saphenous vein graft (2 patients, 20 %), and PTFE interposition grafting (3 patients, 30 %). Six patients also had calf fasciotomies performed prophylactically on the affected lower extremity. The use of tourniquets was utilized in only three patients, including one application by the emergency medical services at the scene. Three patients completed intraoperative angiography at the discretion of the attending surgeon. Two patients (20 %) required emergent abdominal decompression in the postoperative period, within 24 and 72 h of vascular repair. Eight patients (80 %) required a decompressive laparotomy at the completion of lower extremity revascularization.
All surviving patients required ICU admission, with a mean length of stay 9.5 ± 5.4 days (range 4–17 days). Mean length of hospitalization stay was 19.5 ± 11.8 days (range 8–34 days). The overall mortality rate for this series of patients who developed SACS traumatic LEVI was 60 %. Three of these patients arrived with pulseless electrical activity (PEA), and return of hemodynamics was obtained upon fluid resuscitation and ACLS protocol. All patients who arrived in the ED were alive prior to transport to the operating room. Two patients died in the operating room, and four patients died in the ICU secondary to multi-system organ failure. Three of these patients underwent resuscitative thoracotomy in the ED (2 patients) or operating room (1 patient). No significant differences were observed in mortality rates between penetrating and blunt trauma groups.
There were no vascular-related complications, and all vascular repairs were patent upon discharge. Three patients (30 %) required lower extremity amputations during the initial operation for non-salvageable limbs. Complications in the survival group included groin hematoma (10 %) and lower extremity paresthesia (10 %). There were no surgical site infections, and two patients required split-thickness skin grafting to the lower extremity fasciotomy sites.
Discussion
Secondary ACS is yet a poorly understood syndrome where increased intra-abdominal pressure occurs without abdominal injuries. It can be partially explained by two phenomena: massive fluid resuscitation after shock and ischemia–reperfusion injury to the bowel. Nearly all patients developing secondary ACS present with severe shock; in this series mean base deficit at presentation was −17.1 ± 11.2 (range −37 to −2). After this transient period of tissue hypoperfusion, reperfusion with blood flow paradoxically results in further tissue injury due to generation of cytotoxic oxidants [9]. This will ultimately lead to microvascular permeability, and therefore, bowel wall edema [10]. Concurrently, the development of retroperitoneal edema and acute ascites are likely related to resuscitation with large volumes of crystalloid.
Traditionally normal intra-abdominal pressure (IAP) ranges from 2–7 mmHg. According to recent consensus guidelines, IAH is graded as follows: grade I (12–15 mmHg), grade II (16–20 mmHg), grade III (21–25 mmHg) and grade IV (>25 mmHg) [11]. ACS is defined as a sustained IAP greater than 20 mmHg that is associated with new organ dysfunction. The elevated IAP impairs venous return, which further increases capillary filtration pressure, congestion, and edema, thereby leading to organ dysfunction [6].
Recent data suggests that post-injury secondary ACS is an earlier phenomenon, usually manifesting 12–14 h after hospital admission [12]. In this series 80 % of patients developed secondary ACS while in the operating room during the repair of LEVI, while 20 % developed secondary ACS postoperatively in the ICU. Therefore, it is of paramount importance for surveillance of this syndrome to occur in this early time frame, in combination with hemorrhagic control, vascular repair, and completion of diagnostic studies in the pre-ICU period. The clinical suspicion includes elevated peak airway pressure, poor cardiac index, low urinary output, and elevated IAP. The most widely accepted technique of IAP monitoring is via intravesical catheterization. IAP monitoring has been recommended in patients receiving 0.25 L/kg crystalloids, or 10 L of crystalloid (or 10 units of PRBCs) [4, 13]. Biffl et al. [7] recommended IAP monitoring in ICU patients acutely resuscitated from shock (base deficit >10), particularly if requiring vasopressors, and receiving 6 L or more of crystalloid or 6 units of PRBCs in a 6-h period.
The standard treatment of secondary ACS is staged surgical decompression with application of temporary abdominal closure. Critically ill patients on aggressive ventilator support and unstable for transfer to the operating room, can be safely decompressed in the ICU, with standard aseptic techniques [14]. There is no preoperative prognostic factor identified, and a good physiological response to decompression does not ensure better outcome [13, 15]. Cheatham et al. proposed that abdominal perfusion pressure (mean arterial pressure minus IAP) of ≥50 mmHg optimized survival. Balogh et al. [16] correlated persistent response in cardiac output and urinary output with increased outcomes, whereas transient hemodynamic improvement postoperatively was associated with non-survivors. In this series, the mortality was 60 %, which is comparable with other series of trauma patients with similar injury scores, reporting mortality rates of 38–71 % [4, 6, 17]. Interestingly mortality seems to be higher in non-trauma patients admitted in the medical ICU with rates up to 100 % [4]. This may reflect increased awareness of this condition in the trauma population with earlier recognition. It is clear that prompt diagnosis and surgical management aiming at restoration of physiologic status are essential to enhance outcomes. The exact timing and threshold of when decompression should be performed, however, remain unclear [16, 18, 19]. Recent data, including patients decompressed within 16 h of hospital admission, showed no significant difference in mortality in relation to the time until decompression [7].
The key to enhance outcomes of post-injury secondary ACS seems to be prevention. The correct amount corresponds to enough fluid to adequately compensate for losses without over resuscitating. To date there is no accurate parameter to predict third spacing or determine the correct amount of fluid resuscitation. Although not yet widely used, DO2I (oxygen delivery index measures the volume of gaseous oxygen pumped from the left ventricle per minute per meter square and may play a role in determining resuscitation endpoints. Over-resuscitation (DO2I >600 mL/min/m2) does not have additional benefits on the outcome of critically ill patients [20]. Moreover recent data correlated DO2I (at goal 500–600 mL/min/m2) with less crystalloid requirement, better intestinal perfusion, and lower incidence of ACS [21]. Balogh et al. [21] proposed a protocol with goal DO2I at 500 mL/min/m2 during the first 24 h of resuscitation, and to continue resuscitation beyond that time only if evidence of persistent malperfusion. Efforts to maintain a DO2I greater than 500 mL/min/m2 beyond 24 h are rarely beneficial and may be harmful.
We acknowledge that our study has several important limitations. In addition to the retrospective design and relatively small number of patients, we were unable to analyze long-term outcomes, including vascular complications. In our practice, fasciotomies were performed prophylactically at the initial operation immediately after restoration of blood perfusion [22]. The decision to perform fasciotomies was a clinical one and its liberal use has been recommended by some groups [23, 24]. In our previously reported experience, we found fasciotomy rates around 75 % [22], similar to some series [25], but higher as compared to the National Trauma Data Bank of 50 % [26]. The liberal use of fasciotomies appears to be associated with lower rates of amputation. Of the survivors, all vascular grafts remained patent upon discharge, and all laparotomies performed received delayed closures. Interestingly there was no evidence of wound infection among survivors either in abdominal or lower extremity fasciotomy sites.
In conclusion, secondary ACS associated with traumatic lower extremity vascular injuries is associated with very high morbidity and mortality. Large volume fluid resuscitation should be carefully monitored intraoperatively to avoid over-resuscitation in patients at risk for abdominal compartment syndrome. Further studies are needed for identifying causative factors and accurate parameters to prevent such a condition from ensuing.
References
Rodas EB, Malhotra AK, Chhitwal R, et al. Hyperacute abdominal compartment syndrome: an unrecognized complication of massive intraoperative resuscitation for extra-abdominal injuries. Am Surg. 2005;71:977–81.
Cheatum ML, White MW, Sagraves SG, et al. Abdominal perfusion pressure: a superior parameter in the assessment of intra-abdominal hypertension. J Trauma. 2000;49:621–6.
Burch JM, Moore EE, Moore FA, et al. The abdominal compartment syndrome. Surg Clin N Am. 1996;76:833–42.
Maxwell RA, Fabian T, Croce M, et al. Secondary abdominal compartment syndrome: an underappreciated manifestation of severe hemorrhagic shock. J Trauma. 1999;47:995–9.
Madigan MC, Kemp CD, Johnson JC, et al. Secondary abdominal compartment syndrome after severe extremity injury: are early, aggressive fluid resuscitation strategies to blame? J Trauma. 2008;64(2):280–5.
Balogh Z, McKinley BA, Cocanour CS, et al. Secondary abdominal compartment syndrome is an elusive complication of traumatic shock resuscitation. Am J Surg. 2002;184:538–44.
Biffl WL, Moore EE, Burch JM, et al. Secondary abdominal compartment syndrome is a highly lethal event. Am J Surg. 2001;182:645–8.
Offner PJ, de Souza AL, Moore EE, et al. Avoidance of abdominal compartment syndrome in damage-control laparotomy after trauma. Arch Surg. 2001;136:676–81.
Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250:G749–53.
Granger DN, Sennett M, McElearney P, et al. Effect of local arterial hypotension on cat intestinal permeability. Gastroenterology. 1980;79:474–80.
Kirkpatrick AW, Roberts DJ, De Waele J, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definition clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39(7):1190–206.
Dias JJ Jr, Mejia V, Subhawong AP, et al. Protocol for bedside laparotomy in trauma and emergency general surgery: a low return to the operating room. Am Surg. 2005;71:986–91.
Balogh Z, Moore FA, Moore EE, et al. Secondary abdominal compartment syndrome: a potential threat for all trauma clinicians. Injury. 2007;38:272–9.
Ivy ME, Atweh NA, Palmer J, et al. Intraabdominal hypertension and abdominal compartment syndrome in burn patients. J Trauma. 2000;49:387–91.
Kopelman T, Harris C, Miller R, Arrillaga A. Abdominal compartment syndrome in patients with isolated extraperitoneal injuries. J Trauma. 2000;49:744–9.
Balogh Z, McKinley BA, Holcomb JB, et al. Both primary and secondary abdominal compartment syndrome (ACS) can be predicted early and are harbingers of multiple organ failure. J Trauma. 2003;54:848–61.
Ivatury RR, Diebel L, Porter JM, Simon RJ. Intra-abdominal hypertension and abdominal compartment syndrome. Surg Clin N Am. 1997;77(4):783–800.
Ivatury RR, Porter JM, Simon RJ, et al. Intra-abdominal hypertension after life-threatening penetrating abdominal trauma: prophylaxis, incidence, and clinical relevance of gastric mucosal pH and abdominal compartment syndrome. J Trauma. 1998;44:1016–23.
Sugrue M, D’Armours S. The problems with positive end expiratory pressure (PEEP) in association with abdominal compartment syndrome (ACS). J Trauma. 2001;51:419–20.
Velhamos GC, Demetriades D, Shoemaker WC, et al. End-points of resuscitation of critically ill patients: normal or supranormal? A prospective randomized trial. Ann Surg. 2000;232:409–11.
Balogh Z, McKinley BA, Cocanour CS, et al. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg. 2003;138:637–43.
Sciarretta JD, Macedo FI, Ebler DJ, et al. Management of femoral vessels injuries: a 6-year single center experience. Am Surg. 2015;81(1):86–91.
Frykberg ER. Popliteal vascular injuries. Surg Clin N Am. 2002;82(1):67–89.
Feliciano DV, Herskowitz K, O’Gorman RB, et al. Management of vascular injuries of the lower extremities. J Trauma. 1988;28(3):319–28.
Franz RW, Shah KJ, Halaharvi D, et al. A 5-year review of management of lower extremity arterial injuries at an urban level I trauma center. J Vasc Surg. 2011;53:1604–10.
Mullenix PS, Steele SR, Andersen CA, et al. Limb salvage and outcomes among patients with traumatic popliteal vascular injury: an analysis of the National Trauma Data Bank. J Vasc Surg. 2006;44:94–100.
Conflict of interest
F. I. B. Macedo, J. D. Sciarretta, C. A. Otero, G. Ruiz, D. J. Ebler, L. R. Pizano, N. Namias declare that they have no conflict of interest.
Informed consent
Institutional review board approval for this study was obtained, waiving the need for informed consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Macedo, F.I.B., Sciarretta, J.D., Otero, C.A. et al. Secondary abdominal compartment syndrome after complicated traumatic lower extremity vascular injuries. Eur J Trauma Emerg Surg 42, 207–211 (2016). https://doi.org/10.1007/s00068-015-0524-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-015-0524-x