Abstract
Background
Outcome and toxicity profiles of salvage stereotactic ablative radiation strategies for recurrent pre-irradiated brain metastases are poorly defined. This study compared risk–benefit profiles of upfront and salvage iodine-125 brachytherapy (SBT) for small brain metastases. As the applied SBT treatment algorithm required histologic proof of metastatic brain disease in all patients, we additionally aimed to elucidate the value of biopsy before SBT.
Patients and methods
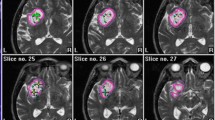
Patients with small untreated (n = 20) or pre-irradiated (n =28) suspected metastases intended for upfront or salvage SBT, respectively, were consecutively included. Temporary iodine-125 implants were used (median reference dose: 50 Gy, median dose rate: 15 cGy/h). Cumulative biologically effective doses (BED) were calculated and used for risk assessment. Treatment toxicity was classified according to Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer (RTOG/EORTC) criteria.
Results
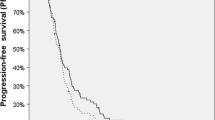
Upfront SBT was initiated in 20 patients and salvage SBT in 23. In 5 patients, salvage SBT was withheld because of proven radiation-induced lesions. Treatment groups exhibited similar epidemiologic data except for tumor size (which was slightly smaller in the salvage group). One-year local/distant tumor control rates after upfront and salvage SBT were similar (94 %/65 % vs. 87 %/57 %, p = 0.45, respectively). Grade I/II toxicity was suffered by 2 patients after salvage SBT (cumulative BED: 192.1 Gy3 and 249.6 Gy3). No toxicity-related risk factors were identified.
Conclusion
SBT combines diagnostic yield with effective treatment in selected patients. The low toxicity rate in the salvage group points to protective radiobiologic characteristics of continuous low-dose rate irradiation. Upfront and salvage SBT are similarly effective and safe. Histologic reevaluation should be reconsidered after previous radiotherapy to avoid under- or overtreatment.
Zusammenfassung
Hintergrund
Daten zu Risiko und Effizienz ablativer stereotaktischer Rebestrahlungsstrategien zerebraler Metastasen sind kaum publiziert worden. Wir verglichen Risiko-Effizienz-Profile der Jod-125 Brachytherapie (SBT) als Primärtherapie kleiner Metastasen und als Rezidivtherapie nach vorausgegangener Bestrahlung. Voraussetzung für eine SBT war immer eine histologische Sicherung der Diagnose. Dieses einheitliche Vorgehen erlaubte, zusätzlich den Stellenwert bioptischer Verfahren zu analysieren.
Patienten und Methoden
Patienten mit kleinen unbehandelten (n = 20) oder vorbestrahlen (n = 28) metastasensuspekten Läsionen wurden konsekutiv eingeschlossen. Implantiert wurden temporäre Jod-125-Seeds (mediane Referenzdosis: 50 Gy, mediane Energiedosisleistung: 15 cGy/h). Für Risikoanalysen berechneten wir die kumulative biologische effektive Dosis (BED). Die Klassifikation der Neurotoxizität erfolgte entsprechend den Kriterien der RTOG/EORTC (Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer).
Ergebnisse
SBT als Initialtherapie wurde bei 20 Patienten und als Rezidivtherapie bei 23 Patienten durchgeführt. Fünf Patienten der Rezidivgruppe wiesen radiogene Gewebsveränderungen auf, sodass keine Behandlung erfolgte. Patienten mit Primär- und Rezidivtherapie waren bezüglich ihrer klinischen Parameter mit Ausnahme des Tumorvolumens vergleichbar (geringfügig kleinere Tumoren in der Rezidivgruppe). Die lokale/distante Einjahrestumorkontrollrate nach Primär- und Rezidiv-SBT war ähnlich (94 %/64 % vs. 87 %/57 %, p = 0,45). Eine Toxizität vom Grad I/II fand sich bei 2 Patienten (kumulative BED: 192,1 Gy3 und 249,6 Gy3).
Schlussfolgerung
SBT verbindet diagnostische Genauigkeit mit effektiver Behandlung. Die niedrige Toxizitätsrate unterstreicht die protektiven radiobiologischen Eigenschaften kontinuierlicher Bestrahlung mit niedriger Energiedosisleistung. SBT als Primär- und Rezidivtherapie ist sicher und effektiv. Die Indikation zur histologischen Reevaluation sollte nach vorausgegangener Bestrahlung immer erwogen werden, um Unter- oder Überbehandlungen zu vermeiden.




Similar content being viewed by others
References
Kocher M, Wittig A, Piroth MD (2014) Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol 190:521–532
Kocher M, Maarouf M, Bendel M (2004) Linac radiosurgery versus whole brain radiotherapy for brain metastases. A survival comparison based on the RTOG recursive partitioning analysis. Strahlenther Onkol 180:263–267
Rades D, Hornung D, Blanck O (2014) Stereotactic radiosurgery for newly diagnosed brain metastases: comparison of three dose levels. Strahlenther Onkol 190:786–791
Treuer H, Hoevels M, Luyken K (2015) Intracranial stereotactic radiosurgery with an adapted linear accelerator vs. robotic radiosurgery: Comparison of dosimetric treatment plan quality. Strahlenther Onkol 191:470–476
Meisner J, Meyer A, Polivka B (2010) Outcome of moderately dosed radiosurgery for limited brain metastases. Report of a single-center experience. Strahlenther Onkol 186:76–81
Ruge MI, Kickingereder P, Grau S (2011) Stereotactic biopsy combined with stereotactic (125)iodine brachytherapy for diagnosis and treatment of locally recurrent single brain metastases. J Neurooncol 105:109–118
Caballero JA, Sneed PK, Lamborn KR (2012) Prognostic factors for survival in patients treated with stereotactic radiosurgery for recurrent brain metastases after prior whole brain radiotherapy. Int J Radiat Oncol Biol Phys 83:303–309
Harris S, Chan MD, Lovato JF (2012) Gamma knife stereotactic radiosurgery as salvage therapy after failure of whole-brain radiotherapy in patients with small-cell lung cancer. Int J Radiat Oncol Biol Phys 83:53–59
Klironomos G, Bernstein M (2013) Salvage stereotactic radiosurgery for brain metastases. Expert Rev Neurother 13:1285–1295
Minniti G, Scaringi C, Paolini S (2016) Repeated stereotactic radiosurgery for patients with progressive brain metastases. J Neurooncol 126:91–97
Malone H, Yang J, Hershman DL, Wright JD (2015) Complications Following Stereotactic Needle Biopsy of Intracranial Tumors. World Neurosurg 84:1084–1089
Schwarz SB, Thon N, Nikolajek K (2012) Iodine-125 brachytherapy for brain tumours-a review. Radiat Oncol 7:30
Dooms GC, Hecht S, Brant-Zawadzki M (1986) Brain radiation lesions: MR imaging. Radiology 158:149–155
Galldiks N, Stoffels G, Filss CP (2012) Role of O‑(2-(18)F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med 53:1367–1374
Ostertag CB, Kreth FW (1995) Interstitial iodine-125 radiosurgery for cerebral metastases. Br J Neurosurg 9:593–503
Schwartz C, Romagna A, Thon N (2015) Outcome and toxicity profile of salvage low-dose-rate iodine-125 stereotactic brachytherapy in recurrent high-grade gliomas. Acta Neurochir (Wien) 157:1757–1764
La Fougère C, Suchorska B, Bartenstein P (2011) Molecular imaging of gliomas with PET: opportunities and limitations. Neuro-oncology 13:806–819
Macdonald DR, Cascino TL, Schold SC Jr (1990) Response criteria for phase II studies of malignant glioma. J Clin Oncol 8:1277–1280
Dale RG (1989) Time-dependent tumour repopulation factors in linear-quadratic equations-implications for treatment strategies. Radiother Oncol 15:371–381
Fowler JF (1989) The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 62:679–694
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31:1341–1346
Patchell RA, Tibbs PA, Regine WF (1998) Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 280:1485–1489
Kocher M, Soffietti R, Abacioglu U (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 29:134–141
Maarouf M, El Majdoub F, Bührle C (2010) Pineal parenchymal tumors: management with interstitial iodine-125 radiosurgery. Strahlenther Onkol 186(3):496–401
Mayer R, Sminia P (2008) Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys 70:1350–1360
Ruge MI, Kocher M, Maarouf M (2011) Comparison of stereotactic brachytherapy (125 iodine seeds) with stereotactic radiosurgery (LINAC) for the treatment of singular cerebral metastases. Strahlenther Onkol 187:7–14
Ruge MI, Suchorska B, Maarouf M (2011) Stereotactic 125iodine brachytherapy for the treatment of singular brain metastases: closing a gap? Neurosurgery 68:1209–1218
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Romagna, C. Schwartz, R. Egensperger, J. Watson, J.-C. Tonn, C. Belka, F.-W. Kreth, and S.B. Nachbichler report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Ethical standards
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Rights and permissions
About this article
Cite this article
Romagna, A., Schwartz, C., Egensperger, R. et al. Iodine-125 brachytherapy as upfront and salvage treatment for brain metastases. Strahlenther Onkol 192, 780–788 (2016). https://doi.org/10.1007/s00066-016-1009-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-016-1009-5