Abstract
Aim
Microvascular free tissue transfer is a standard method in head and neck reconstructive surgery. However, previous radiotherapy of the operative region is associated with an increased incidence in postoperative flap-related complications and complete flap loss. As transforming growth factor beta (TGF-β) 1 and galectin-3 are well known markers in the context of fibrosis and lectin-like oxidized low-density lipoprotein 1 (LOX-1) supports vascular atherosclerosis, the aim of this study was to evaluate the expression of TGF-β1 and related markers as well as LOX-1 in irradiated vessels.
Materials and methods
To evaluate the expression of galectin-3, Smad 2/3, TGF-β1, and LOX-1, 20 irradiated and 20 nonirradiated arterial vessels were used for immunohistochemical staining. We semiquantitatively assessed the ratio of stained cells/total number of cells (labeling index).
Results
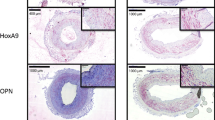
Expression of galectin-3, Smad 2/3, and TGF-β1 was significantly increased in previously irradiated vessels compared with nonirradiated controls. Furthermore, LOX-1 was expressed significantly higher in irradiated compared with nonirradiated vessels.
Conclusion
Fibrosis-related proteins like galectin-3, Smad 2/3, and TGF-β1 are upregulated after radiotherapy and support histopathological changes leading to vasculopathy of the irradiated vessels. Furthermore, postoperative complications in irradiated patients can be explained by increased endothelial dysfunction caused by LOX-1 in previously irradiated patients. Consequently, not only TGF-β1 but also galectin-3inhibitors may decrease complications after microsurgical tissue transfer.
Zusammenfassung
Einführung
Der freie mikrovaskuläre Gewebetransfer gilt heute als fester Standard in der rekonstruktiven Kopf-Hals-Chirurgie. Es zeigte sich jedoch, dass im Falle einer stattgehabten Bestrahlung im Operationsgebiet mit einer erhöhten Rate an transplantatbezogenen Komplikationen gerechnet werden muss. Sowohl TGF-β1 als auch Galektin-3 sind bekannte Mediatoren in Bezug auf die Fibroseentstehung, wohingegen LOX-1 eine unterstützende Rolle bei der Entwicklung von Atherosklerose einnimmt. Das Ziel dieser Studie war es, zu untersuchen, wie sich die Expression von TGF-β1 und verwandten Fibrosemediatoren sowie LOX-1 im bestrahlten Gefäß verhält.
Material und Methoden
20 bestrahlte und 20 nichtbestrahlte Arterien wurden mittels immunhistochemischer Färbungen auf die Expression von Galektin-3, Smad2/3, TGF-β1 sowie LOX-1 hin untersucht. Semiquantitativ wurde das Verhältnis gefärbte Zellen pro Gesamtzellzahl (Färbungsindex) bestimmt.
Ergebnisse
Die Expression von Galektin-3, Smad2/3 sowie TGF-β1 war in bestrahlten Arterien signifikant höher als in nichtbestrahlten Gefäßen. LOX-1 wurde in vorbestrahlten Gefäßen ebenso signifikant höher exprimiert.
Konklusion
Mediatoren der Fibroseentstehung wie Galektin-3, Smad2/3 und TGF-β1 sind in bestrahlten Gefäßen erhöht exprimiert und tragen mutmaßlich zu der Entstehung der radiogen induzierten Vaskulopathie bei. Des Weiteren können postoperative Komplikationen bei bestrahlen Patienten durch die verstärkte endotheliale Dysfunktion, unterstützt durch LOX-1, erklärt werden. Folglich könnten nicht nur TGF-β1- sondern auch Galektin-3-Inhibitoren einen minimierenden Effekt auf die Komplikationen nach mikrovaskulärem Gewebetransfer haben.





Similar content being viewed by others
References
Baluna RG, Eng TY, Thomas CR (2006) Adhesion molecules in radiotherapy. Radiat Res 166:819–831
Beckman JA, Thakore A, Kalinowski BH et al (2001) Radiation therapy impairs endothelium-dependent vasodilation in humans. J Am Coll Cardiol 37:761–765
Benatar MJ, Dassonville O, Chamorey E et al (2013) Impact of preoperative radiotherapy on head and neck free flap reconstruction: a report on 429 cases. J Plast Reconstr Aesthet Surg 66:478–482
Dorresteijn LD, Kappelle AC, Scholz NM et al (2005) Increased carotid wall thickening after radiotherapy on the neck. Eur J Cancer 41:1026–1030
Halle M, Gabrielsen A, Paulsson-Berne G et al (2010) Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 55:1227–1236
Halle M, Hall P, Tornvall P (2011) Cardiovascular disease associated with radiotherapy: activation of nuclear factor kappa-B. J Intern Med 269:469–477
Hinagata J, Kakutani M, Fujii T et al (2006) Oxidized LDL receptor LOX-1 is involved in neointimal hyperplasia after balloon arterial injury in a rat model. Cardiovasc Res 69:263–271
Holler V, Buard V, Gaugler MH et al (2009) Pravastatin limits radiation-induced vascular dysfunction in the skin. J Invest Dermatol 129:1280–1291
Kasper M, Fehrenbach H (2000) Immunohistochemical evidence for the occurrence of similar epithelial phenotypes during lung development and radiation-induced fibrogenesis. Int J Radiat Biol 76:493–501
Kume N, Kita T (2001) Roles of lectin-like oxidized LDL receptor-1 and its soluble forms in atherogenesis. Curr Opin Lipidol 12:419–423
Li DY, Chen HJ, Staples ED et al (2002) Oxidized low-density lipoprotein receptor LOX-1 and apoptosis in human atherosclerotic lesions. J Cardiovasc Pharmacol Ther 7:147–153
Li JH, Huang XR, Zhu HJ et al (2004) Advanced glycation end products activate Smad signaling via TGF-beta-dependent and independent mechanisms: implications for diabetic renal and vascular disease. FASEB J 18:176–178
Mackinnon AC, Gibbons MA, Farnworth SL et al (2012) Regulation of transforming growth factor-beta1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med 185:537–546
Martin M, Lefaix JL, Pinton P et al (1993) Temporal modulation of TGF-beta 1 and beta-actin gene expression in pig skin and muscular fibrosis after ionizing radiation. Radiat Res 134:63–70
Mucke T, Rau A, Weitz J et al (2012) Influence of irradiation and oncologic surgery on head and neck microsurgical reconstructions. Oral Oncol 48:367–371
Pohlenz P, Klatt J, Schon G et al (2012) Microvascular free flaps in head and neck surgery: complications and outcome of 1000 flaps. Int J Oral Maxillofac Surg 41:739–743
Rodriguez-Vita J, Sanchez-Lopez E, Esteban V et al (2005) Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation 111:2509–2517
Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E et al (2007) TGF-beta signaling in vascular fibrosis. Cardiovasc Res 74:196–206
Russell NS, Hoving S, Heeneman S et al (2009) Novel insights into pathological changes in muscular arteries of radiotherapy patients. Radiother Oncol 92:477–483
Saito A, Fujimura M, Inoue T et al (2010) Relationship between lectin-like oxidized low-density lipoprotein receptor 1 expression and preoperative echogenic findings of vulnerable carotid plaque. Acta Neurochi 152:589–595
Schultze-Mosgau S, Erbe M, Keilholz L et al (2000) Histomorphometric analysis of irradiated recipient vessels and transplant vessels of free flaps in patients undergoing reconstruction after ablative surgery. Int J Oral Maxillofac Surg 29:112–118
Schultze-Mosgau S, Wehrhan F, Grabenbauer G et al (2002) Transforming growth factor beta1 and beta2 (TGFbeta2/TGFbeta2) profile changes in previously irradiated free flap beds. Head Neck 24:33–41
Schultze-Mosgau S, Wehrhan F, Amann K et al (2003) In Vivo TGF-beta 3 expression during wound healing in irradiated tissue. An experimental study. Strahlenther Onkol 179:410–416
Schultze-Mosgau S, Blaese MA, Grabenbauer G et al (2004) Smad-3 and Smad-7 expression following anti-transforming growth factor beta 1 (TGFbeta1)-treatment in irradiated rat tissue. Radiother Oncol 70:249–259
Scott AS, Parr LA, Johnstone PA (2009) Risk of cerebrovascular events after neck and supraclavicular radiotherapy: a systematic review. Radiother Oncol 90:163–165
Sugihara T, Hattori Y, Yamamoto Y et al (1999) Preferential impairment of nitric oxide-mediated endothelium-dependent relaxation in human cervical arteries after irradiation. Circulation 100:635–641
Wehrhan F, Grabenbauer GG, Rodel F et al (2004) Exogenous modulation of TGF-beta(1) influences TGF-betaR-III-associated vascularization during wound healing in irradiated tissue. Strahlenther Onkol 180:526–533
Acknowledgments
Author contribution
R.H.M.P., P.M., A.S. and F.W. designed the study, R.H.M.P., P.M. and M.W. collected the data, R.H.M.P., F.W., K.A., A.S. and F.N. drafted the article and all authors approved the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R.H.M. Preidl, P. Möbius, M. Weber, K. Amann, F.W. Neukam, A. Schlegel, and F. Wehrhan state that there are no conflicts of interest. All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Additional information
Raimund H.M. Preidl and Patrick Möbius shared first authorship.
Rights and permissions
About this article
Cite this article
Preidl, R., Möbius, P., Weber, M. et al. Expression of transforming growth factor beta 1-related signaling proteins in irradiated vessels. Strahlenther Onkol 191, 518–524 (2015). https://doi.org/10.1007/s00066-014-0797-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0797-8
Keywords
- Radiotherapy
- Vessel
- Radiation-induced vasculopathy
- Transforming growth factor beta
- Head and neck surgery