Abstract
Purpose
The purpose of this work was to investigate the prognostic value of response analysis using early 3’-deoxy-3’-[18F]-fluorothymidine (18F-FLT) PET/CT in esophageal squamous cancer patients and make a comparison with [18F]-fluorodeoxyglucose (18F-FDG) PET/CT.
Patients and materials
For 34 patients with esophageal squamous cell cancer, both 18F-FLT PET/CT and 18F-FDG PET/CT scans were performed at baseline (pre), 4 weeks after the start of radiotherapy or chemoradiotherapy (interim), and 2 weeks after therapy completion (final). SUVmax1, SUVmax2, and SUVmax3 represent SUVmax (SUV: standard uptake values) measured on the pre, interim, and final scans, respectively. GTVFLT-PET and GTVFDG-PET (GTV: gross tumor volume) were measured on the pre and interim scans. ΔSUV/ΔGTV represents the fractional changes of SUVmax/GTV between two different time points. PET parameters were evaluated for correlations with outcome.
Results
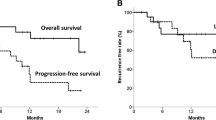
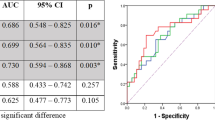
Regarding 18F-FLT PET/CT, according to receiver operating characteristic (ROC) curve analysis, parameters for predicting 2-year progression-free survival (PFS) and locoregional control (LRC) showed the highest area under curve (AUC) on interim 18F-FLT PET/CT scans (ΔSUV12, AUC of 0.812 for PFS, 0.775 for LRC, with a cutoff of 60 %; P = 0.008), compared with the parameters on pre and final scans. Patients with a ΔSUV12 greater than 60 %, who were defined as interim PET-negative group, were associated with better 2-year PFS and LRC than the interim PET-positive group (PFS: 70.6 % vs. 35.2 %, P = 0.025; LRC: 84.2 % vs 52.9, P = 0.046). In terms of 18F-FDG PET/CT, ΔSUV13 on the final 18F-FDG PET/CT scan demonstrated better prediction (AUC of 0.812 for PFS, 0.807 for LRC, with a cutoff of 75 %; P = 0.016) than the parameters on pre and interim scans. An SUVmax decrease ≥ 75 % on the final 18F-FDG PET/CT scan was associated with better clinical outcome (PFS: 73.3 % vs. 36.8 %, P = 0.022; LRC: 86.7 % vs 52.6, P = 0.029). These correlations were most prominent in the subgroup of patients treated with chemoradiotherapy.
Conclusion
Early interim 18F-FLT PET/CT is a significant predictor of 2-year PFS and LRC, which is correlated better with early responses and late outcomes than interim 18F-FDG PET/CT in esophageal squamous cancer patients.
Zusammenfassung
Ziel
Das Ziel der Arbeit war es, die Vorhersage der biologischen Tumorantwortmit einer frühen [18F]-Fluorothymidin(FLT)-PET/CT bei mit Radiochemotherapie behandelten Patienten mit einem Plattenepithelkarzinom des Ösophagus zu untersuchen und die Ergebnisse mit der [18F]-Fluorodeoxyglukose(FDG)-PET/CT zu vergleichen.
Patienten und Methodik
Bei 34 Patienten mit einem Plattenepithelkarzinom des Ösophagus, wurden beide [18F]-FLT-PET/CT- und [18F]-FDG-PET/CT-Untersuchungen jeweils zu Beginn, 4 Wochen nach Beginn der Strahlen- oder Radiochemotherapie und 2 Wochen nach Therapieabschluss durchgeführt. SUVmax1, SUVmax2 und SUVmax3 (SUV: „standard uptake values“) entsprechen der gemessenen SUVmax vor-, zwischen- und nach der Strahlen- oder Radiochemotherapie. Das makroskopische Tumorvolumen (GTV: „gross tumor volume“) GTVFLT-PET und GTVFDG-PET wurde anhand der PET/CT-Ergebnisse vor und während der Strahlentherapie vollzogen. ΔSUV/ΔGTV entspricht der Änderungsrate von SUVmax/GTV zwischen den beiden unterschiedlichen Zeitpunkten. Es wurde die Korrelation der PET/CT-Parameter mit dem Outcome analysiert.
Ergebnisse
Die Analyse der ROC-(Receiver-Operating-Characteristic-Kurve- zeigte, dass im Vergleich zu den Untersuchungen vor- und nach Strahlentherapie während der [18F]-FLT-PET/CT die höchste Fläche unter der Kurve („area under the curve“, AUC) für die Parameter zur Vorhersage des progressionsfreien 2-Jahres-Überlebens (PFS) und der lokalen Kontrolle (LRC) festgestellt wurden (ΔSUV12, AUC von 0,812 für PFS und 0,775 für LRC, bei einem Grenzwert von 60 %; P = 0,008) . Patienten mit einem ΔSUV12 größer als 60 %, die als PET-negative Patienten definiert wurden, hatten eine höhere LKC und ein besseres 2-Jahres-PFS als PET-positive Patienten (LRC: 84,2 % vs. 52,9; P = 0,046; PFS: 70,6 % vs. 35,2 %; P = 0,025). Die Analyse der [18F]-FDG-PET/CT ergab, dass ΔSUV13 bei der PET/CT nach Strahlentherapie eine bessere Vorhersage der Prognose ermöglicht, als die PET/CT vor- und während der Strahlentherapie (AUC 0,812 für PFS; 0,807 für LRC, bei einem Grenzwert von 75 %; P = 0,016). Eine Reduktion der SUVmax von mehr als 75 % warmit einem besseren klinischen Outcome der Patienten assoziiert (PFS: 73,3 % vs. 36,8 %; P = 0,022; LRC: 86,7 % vs. 52,6; P = 0,029). Für die Subgruppe der Patienten, die mit einer Chemoradiotherapie behandelt wurden, waren diese Korrelationen deutlicher.
Schlussfolgerungen
Die frühe [18F]-FLT-PET/CT während der Strahlentherapie ist ein signifikanter Prädiktor für das 2-Jahres-PFS und die LRC und korreliert bei Plattenepithelkarzinomen des Ösophagus im Vergleich zur [18F]-FDG-PET/CT besser mit einem frühem Ansprechen und einem späten Outcome.




Similar content being viewed by others
References
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24:2137–2150
Semrau R, Herzog SL, Vallbohmer D et al (2012) Radiotherapy in elderly patients with inoperable esophageal cancer. Is there a benefit? Strahlenther Onkol 188:226–232
Westerterp M, van Westreenen HL, Reitsma JB et al (2005) Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy–systematic review. Radiology 236:841–851
Tixier F, Le Rest CC, Hatt M et al (2011) Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med 52:369–378
Essler M, Wantke J, Mayer B et al (2013) Positron-emission tomography CT to identify local recurrence in stage I lung cancer patients 1 year after stereotactic body radiation therapy. Strahlenther Onkol 189:495–501
Troost EG, Schinagl DA, Bussink J et al (2010) Innovations in radiotherapy planning of head and neck cancers: role of PET. J Nucl Med 51:66–76
Arslan N, Miller TR, Dehdashti F et al (2002) Evaluation of response to neoadjuvant therapy by quantitative 2-deoxy-2-[18F]fluoro-D-glucose with positron emission tomography in patients with esophageal cancer. Mol Imaging Biol 4:301–310
Jadvar H (2013) Imaging evaluation of prostate cancer with 18F-fluorodeoxyglucose PET/CT: utility and limitations. Eur J Nucl Med Mol Imaging 40:5–10
Yang YJ, Ryu JS, Kim SY et al (2006) Use of 3’-deoxy-3’-[18F]fluorothymidine PET to monitor early responses to radiation therapy in murine SCCVII tumors. Eur J Nucl Med Mol Imaging 33:412–419
Herrmann K, Buck AK, Schuster T et al (2011) Predictive value of initial 18F-FLT uptake in patients with aggressive non-Hodgkin lymphoma receiving R-CHOP treatment. J Nucl Med 52:690–696
Lee H, Kim SK, Kim YI et al (2014) Early determination of prognosis by interim 3’-deoxy-3’-18F-fluorothymidine PET in patients with non-Hodgkin lymphoma. J Nucl Med 55:216–222
Machulla H-J, Blocher A, Kuntzsch M et al (2000) Simplified labeling approach for synthesizing 3’-deoxy-3’-[18F] fluorothymidine ([18F] FLT). J Radioanal Nucl Chem 243:843–846
Kim JJ, Tannock IF (2005) Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer 5:516–525
Withers HR, Taylor JM (1993) Critical volume model. Int J Radiat Oncol Biol Phys 25:151–152
Cvek J, Kubes J, Skacelikova E et al (2012) Hyperfractionated accelerated radiotherapy with concomitant integrated boost of 70–75 Gy in 5 weeks for advanced head and neck cancer. A phase I dose escalation study. Strahlenther Onkol 188:666–670
Balermpas P, Bauer C, Fraunholz I et al (2014) Concomitant chemoradiotherapy versus induction chemotherapy followed by chemoradiotherapy as definitive, first line treatment of squamous cell carcinoma of the head and neck. A retrospective single center analysis. Strahlenther Onkol 190:256–262
Bonner JA, Harari PM, Giralt J et al (2010) Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 11:21–28
Murphy JD, La TH, Chu K et al (2011) Postradiation metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys 80:514–521
Castaldi P, Rufini V, Bussu F et al (2012) Can “early” and “late”18F-FDG PET-CT be used as prognostic factors for the clinical outcome of patients with locally advanced head and neck cancer treated with radio-chemotherapy? Radiother Oncol 103:63–68
Lordick F, Ott K, Krause BJ et al (2007) PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 8:797–805
zum Buschenfelde CM, Herrmann, Schuster T et al (2011) (18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial. J Nucl Med 52:1189–1196
Ott K, Weber WA, Lordick F et al (2006) Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol 24:4692–4698
Lin Q, Yang R, Sun L et al (2012) Biological response of nasopharyngeal carcinoma to radiation therapy: a pilot study using serial 18F-FDG PET/CT scans. Cancer Invest 30:528–536
Sohn HJ, Yang YJ, Ryu JS et al (2008) [18F]Fluorothymidine positron emission tomography before and 7 days after gefitinib treatment predicts response in patients with advanced adenocarcinoma of the lung. Clin Cancer Res 14:7423–7429
Hoeben BA, Troost EG, Span PN et al (2013) 18F-FLT PET during radiotherapy or chemoradiotherapy in head and neck squamous cell carcinoma is an early predictor of outcome. J Nucl Med 54:532–540
Arens AI, Troost EG, Hoeben BA et al (2014) Semiautomatic methods for segmentation of the proliferative tumour volume on sequential FLT PET/CT images in head and neck carcinomas and their relation to clinical outcome. Eur J Nucl Med Mol Imaging 41:915–924
Schwarzenberg J, Czernin J, Cloughesy TF et al (2012) 3’-deoxy-3’-18F-fluorothymidine PET and MRI for early survival predictions in patients with recurrent malignant glioma treated with bevacizumab. J Nucl Med 53:29–36
Yue J, Chen L, Cabrera AR et al (2010) Measuring tumor cell proliferation with 18F-FLT PET during radiotherapy of esophageal squamous cell carcinoma: a pilot clinical study. J Nucl Med 51:528–534
Pastor C, Subtil JC, Sola J et al (2011) Accuracy of endoscopic ultrasound to assess tumor response after neoadjuvant treatment in rectal cancer: can we trust the findings? Dis Colon Rectum 54:1141–1146
Compliance with ethical guidelines
Acknowledgements
This study was supported by grant No. 81101067 from National Nature Science Foundation of China.
Conflict of interest
H. Chen, Y. Li, H. Wu, L. Sun, Q. Lin, L. Zhao and H. An state that there are no conflicts of interest.
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Consent was obtained from all patients identifiable from images or other information within the manuscript. In the case of underage patients, consent was obtained from a parent or legal guardian.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Haojun Chen and Yimin Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, H., Li, Y., Wu, H. et al. 3’-Deoxy-3’-[18F]-fluorothymidine PET/CT in early determination of prognosis in patients with esophageal squamous cell cancer. Strahlenther Onkol 191, 141–152 (2015). https://doi.org/10.1007/s00066-014-0744-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0744-8