Abstract
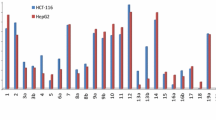
Novel thiophene derivatives 3a–f were synthesized by using the 3-oxo-3-propanenitrile derivatives 1a–c with triethylamine, elemental sulfur and either of malononitrile or ethyl cyanoacetate. Compounds 3a, 3c, and 3e were reacted with ethyl cyanoacetate to give the 2-(N-cyanoacetamido)-thiophene derivatives 4a–f. The latter compounds underwent ready cyclization in sodium ethoxide to give the thieno[2,3-b]pyridine derivatives 5a–f. Compounds 5a–f underwent [4 + 2] cycloaddtion to produce the quinoline derivatives 7a–f. The newly synthesized products were assessed for antitumor activity towards human cancer human gastric cancer (NUGC and HR), human colon cancer (DLD1), human liver cancer (HA22T and HEPG2), human breast cancer (MCF), nasopharyngeal carcinoma (HONE1) and normal fibroblast (WI38) cell lines. Excellent antitumor activities were shown by compounds 3c, 3d, 4b, 5b, 8c, 8d, 9a, 9c, 9d, 11d, and 15d, where they exhibited optimal cytotoxic effect against the cancer cell lines, with IC50s in the nM range. Compounds 11d and 3c showed the maximum inhibitory effect and these are much higher than the reference CHS-828.




Similar content being viewed by others
References
Barnes DM, Haight AR, Hameury T, McLaughlin MA, Mei J, Tedrow JS, Toma JDR (2006) New conditions for the synthesis of thiophenes via the Knoevenagel/Gewald reaction sequence. Application to the synthesis of a multi-targeted kinase inhibitor. 62:11311–11319
Bozorov K, Ma HR, Zhao JY, Zhao HQ, Chen H, Bobakulov K, Xin XL, Elmuradov B, Shakhidoyatov K, Aisa HA (2014) Discovery of diethyl 2,5-diaminothiophene-3,4-dicarboxylate derivatives as potent anticancer and antimicrobial agents and screening of anti-diabetic activity: synthesis and in vitro biological evaluation. Eur J Med Chem 84:739–745
Calleja MC, Persoone G (1992) The potential of ecotoxicological tests for the prediction of acute toxicity in man as evaluated on the first ten chemicals of the M.E.I.C. programme. Atla 20:396–405
Carballo JL, Hernández-Inda ZL, Pérez P, Grávalos MDG (2002) A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol 2(17):1–5
Chen HH, Gross S, Liao J, McLaughlin M, Dean T, Sly WS, Jesse A, May JA (2000) 2H-Thieno[3,2-e]- and [2,3-e]-1,2-thiazine-6-sulfonamide 1,1-dioxides as ocular hypotensive agents: synthesis, carbonic anhydrase inhibition and evaluation in the rabbit. Biorg Med Chem 8:957–975
Choudhary MI, Thomsen WJ (2001) Bioassay Techniques For Drug Development, Harwood Academic Publishers, UK, pp 9–1
Davari AS, Abnous K, Mehri S, Ghandadi M, Hadizadeh F (2014) Synthesis and biological evaluation of novel pyridine derivatives as potential anticancer agents and phosphodiesterase-3 inhibitors. Bioorg Chem 57:83–89
Dehbi O, Tikad A, Bourg S, Bonnet P, Lozach O, Meijer L, Aadil M, Akssira M, Guillaumet G, Routier S (2014) Synthesis and optimization of an original V-shaped collection of 4-7-disubstituted Pyrido[3,2-d]pyrimidines as CDK5 and DYRK1A inhibitors. Eur J Med Chem 80:352–363
Fakhr MII, Radwan MAA, El-Batran S, Abd El-Salam OME, El-Shenawy SM (2009) Synthesis and pharmacological evaluation of 2-substituted benzo[b]thiophenes as anti-inflammatory and analgesic agents. Eur J Med Chem 44:1718–1725
Guo Z, Chen Y, Wu D, Zhu YF, Struthers RS, Saunders J, Xie Q, Chen C (2003) Synthesis and structure–Activity relationships of thieno[2,3-d]pyrimidine-2,4-dione derivatives as potent GnRH receptor antagonists. Bioorg Med Chem 13:3617–3622
Harvey AL, Young LC, Kornisiuk E, Snitcofsky M, Colettis N, Blanco C, Jerusalinsky D, Jamieson AG, Hartley RC, Stone TW (2012) Novel dihydro-pyrazolo[3,4-d](1,2,4)triazolo(1,5a)pyrimidin-4-one (AJ23) is an antagonist at adenosine A1 receptors and enhances consolidation of step-down avoidance. Behav Brain Res 234:184–191
Helal MH, El-Awdan SA, Salem MA, Abd-Elaziz TA, Moahamed YA, El-Sherif AA, Mohamed GAM (2015) Synthesis, biological evaluation and molecular modeling of novel series of pyridine derivatives as anticancer, anti-inflammatory and analgesic agents. Spectrochim Acta 135:764–773
Kadayat TM, CPark C, Jun KY, Magar TBT, Bist G, Yoo HY, Kwon Y, Seok E (2015) Design and synthesis of novel 2,4-diaryl-5H-indeno[1,2-b]pyridine derivatives, and their evaluation of topoisomerase inhibitory activity and cytotoxicity. Bioorg Med Chem 23:160–173
Kandeel MM, Refaat HM, Kassab AE, Shahin IG, Abdelghany TM (2015) Synthesis, anticancer activity and effects on cell cycle profile and apoptosis of novel thieno[2,3-d]pyrimidine and thieno[3,2-e] triazolo[4,3-c]pyrimidine derivatives. Eur J Med Chem 90:920–632
Ma LY, Wang B, Pang LP, Zhang M, Wang SQ, Zheng YC, Shao KP, Xue DQ, Liu HM (2015) Design and synthesis of novel 1,2,3-triazole–pyrimidine–urea hybrids as potential anticancer agents. Bioorg Med Chem Lett 25:1124–1128
Martorana M, Gentile C, Perricone U, Piccionello AP, Bartolotta R, Terenzi l, Pace A, Mingoia F, Almerico AM, Lauria A (2015) Synthesis, antiproliferative activity, and in silico insights of new 3-benzoylamino-benzo[b]thiophene derivatives. Eur J Med Chem 90:537–546
Pottoo FH, Bhowmik M, Vohora D (2014) Raloxifene protects against seizures and neurodegeneration in a mouse model mimicking epilepsy in postmenopausal woman. Eur J of Pharm Scie 65:167–173
Shi W, Marcus SL, Lowary TL (2011) Cytotoxicity and topoisomerase I/II inhibition of glycosylated 2-phenyl-indoles, 2-phenyl-benzo[b]thiophenes and 2-phenyl-benzo[b]furans. Bioorg Med Chem 19:603–612
Wu L, Zhang C, Li W (2013) Regioselective synthesis of 6-aryl-benzo[h][1,2,4]-triazolo[5,1-b]quinazoline-7,8-diones as potent antitumoral agents. Bioorg Med Chem Lett 23:5002–5005
Zeng XX, Zheng RL, Zhou T, He HY, Liu JY, Zheng Y, Tong AP, Xiang ML, Song XR, Yang SY, Yu L, Wei YQ, Zhao YL, Yang L (2010) Novel thienopyridine derivatives as specific anti-hepatocellular carcinoma (HCC) agents: Synthesis, preliminary structure–activity relationships, and in vitro biological evaluation. Bioorg Med Chem Lett 20:6282–6285
Acknowledgements
R. M. Mohareb would like to thank the Alexander von Humboldt Foundation in Bonn, germany for affording him regular fellowships in Germany for finance and completing his research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest
Rights and permissions
About this article
Cite this article
Mohareb, R.M., Ibrahim, R.A. Design, cytotoxicity and toxicity of new thiophene and thieno [2,3-b] pyridine derivatives. Med Chem Res 26, 587–602 (2017). https://doi.org/10.1007/s00044-017-1780-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1780-6