Abstract
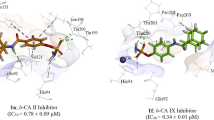
A series of novel compounds bearing substituted pyrrolidinone moieties at the para-position of benzenesulfonamide was synthesized as inhibitors of carbonic anhydrase (CA) isoform IX, known to be overexpressed in numerous human cancers. The inhibitors were tested towards all twelve catalytically active human CA isozymes to determine the affinity and selectivity towards CA IX over other isoforms. Compound affinity was determined by the fluorescence-based thermal shift assay and verified by isothermal titration calorimetry. Several compounds exhibited nanomolar binding affinity against CA IX while significantly weaker binding to the cytosolic off-target CA I was observed. The most effective CA IX binders reached the K d of about 50 nM. This series of compounds can be used for further development of inhibitors with significant binding affinity and selectivity towards anticancer CA IX isozyme.





Similar content being viewed by others
References
Anusevicius K, Vaickelioniene R, Mickevicius V, Mikulskiene G (2013) Synthesis of bis azole, diazole,triazole derivatives N-(4-chloro/iodophenyl)-N-carboxyethyl-β-alanine dihydrazides. J Heterocycl Chem 50:309–314
Aspatwar A, Tolvanen ME, Ortutay C, Parkkila S (2014) Carbonic anhydrase related proteins: molecular biology and evolution. Subcell Biochem 75:135–156
Baranauskienė L, Hilvo M, Matulienė J, Golovenko D, Manakova E, Dudutienė V, Michailovienė V, Torresan J, Jachno J, Parkilla S, Maresca A, Supuran CT, Gražulis S, Matulis D (2010) Inhibition and binding studies of carbonic anhydrase isozymes I,II and IX with benzimidazo[1,2c][1,2,3]thiadiazole-7-sulphonamides. J Enzyme Inhib Med Chem 25:863–870
Cimmperman P, Baranauskienė L, Jachimovičiūtė S, Jachno J, Torresan J, Michailovienė V, Matulienė J, Sereikaitė J, Bumelis V, Matulis D (2008) A quantitative model of thermal stabilization and destabilization of proteins by ligands. Biophys J 95:3222–3231
Dudutienė V, Matulienė J, Smirnov A, Timm DD, Zubrienė A, Baranauskienė L, Morkūnaitė V et al. (2014) Discovery and characterization of novel selective inhibitors of carbonic anhydrase IX. J Med Chem 57:9435–9446
Hassan MI, Shajee B, Waheed A, Ahmad F, Sly WS (2013) Structure, function and applications of carbonic anhydrase isozymes. Bioorg Med Chem 21:1570–1582
Krasavin M, Korsakov M, Dorogov M, Tuccinardi T, Dedeoglu N, Supuran CT (2015) Probing the ‘bipolar’ nature of the carbonic anhydrase active site: aromatic sulfonamides containing 1,3-oxazol-5-yl moiety as picomolar inhibitors of cytosolic CA I and CA II isoforms. Eur J Med Chem 101:334–347
Lock FE, McDonald PC, Lou Y, Serrano I, Chafe SC, Ostlund C, Aparicio S, Winum JY, Supuran CT, Dedhar S (2013) Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene 32:5210–5219
McDonald PC, Winum JY, Supuran CT, Dedhar S (2012) Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget 3:84–97
Monti SM, Supuran CT, De Simone G (2013) Anticancer carbonic anhydrase inhibitors: a patent review (2008–2013). Expert Opin Ther Patents 23:737–749
Moser KM, Luchsinger PC, Adamson JS, McMahon JS, Schlueter DP, Spivack M, Weg JG (1973) Respiratory stimulation with intravenous doxapram in respiratory failure. A double-blind co-operative study. N Engl J Med 288:427–431
Moutevelis-Minakakis P, Papavassilopoulou E, Michas G, Georgikopoulou K, Ragoussi ME, Neophytou N, Zoumpoulakis P, Mavromoustakos T, Hadjipavlou-Litina D (2011) Synthesis, in silico docking experiments of new 2-pyrrolidinone derivatives and study of their anti-inflammatory activity. Bioorg Med Chem 19:2888–2902
Pacchiano F, Aggarwal M, Avvaru BS, Robbins AH, Scozzafava A, McKenna R, Supuran CT (2010) Selective hydrophobic pocket binding observed within the carbonic anhydrase II active site accommodate different 4-substituted-ureido-benzenesulfonamides and correlate to inhibitor potency. Chem Commun (Camb) 46:8371–8373
Pacchiano F, Carta F, McDonald PC, Lou Y, Vullo D, Scozzafava A, Dedhar S, Supuran CT (2011) Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem 54:1896–1902
Pastorekova S, Parkkila S, Zavada J (2006) Tumor-associated carbonic anhydrases and their clinical significance. Adv Clin Chem 42:167–216
Rami M, Dubois L, Parvathaneni NK, Alterio V, van Kuijk SJA, Monti SM, Lambin P, De Simone G, Supuran CT, Winum JY (2013) Hypoxia-targeting carbonic anhydrase IX inhibitors by a new series of nitroimidazole-sulfonamides/sulfamides/sulfamates. J Med Chem 56:8512–8520
Rutkauskas K, Zubrienė A, Tumosienė I, Kantminienė K, Kažemėkaitė M, Smirnov A, Kazokaitė J, Morkūnaitė V, Čapkauskaitė E, Matulis D (2014) 4-Amino-substituted benzenesulfonamides as inhibitors of human carbonic anhydrases. Molecules 19:17356–17380
Sedlakova O, Svastova E, Takacova M, Kopacek J, Pastorek J, Pastorekova S (2014) Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front Physiol 4:400
Siddiqui N, Ahsan W, Alam MS, Ali R, Srivastava K (2012) Design, synthesis and evaluation of anticonvulsant activity of pyridinyl-pyrrolidones: a pharmacophore hybrid approach. Arch Pharm (Weinheim) 345:185–194
Strelciunaite V, Anusevicius K, Tumosiene I, Siugzdaite J, Jonuskiene I, Ramanauskaite I, Mickevicius V (2016) Synthesis of novel benzimidazoles 2-functionalized with pyrrolidinone and γ-amino acid with a high antibacterial activity. Heterocycles 92:235–251
Supuran CT (2008) Carbonic anhydrases–an overview. Curr Pharm Des 14:603–614
Supuran CT (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 7:168–181
Supuran CT, Winum JY (2015) Carbonic anhydrase IX inhibitors in cancer therapy: an update. Future Med Chem 7:1407–1414
Supuran CT, Winum JY (2015) Designing carbonic anhydrase inhibitors for the treatment of breast cancer. Expert Opin Drug Discov 10:591–597
Tafreshi NK, Bui MM, Bishop K, Lloyd MC, Enkemann SA, Lopez AS, Abrahams D et al. (2012) Noninvasive detection of breast cancer lymph node metastasis using carbonic anhydrases IX and XII targeted imaging probes. Clin Cancer Res 18:207–219
Tanpure RP, Ren B, Peat TS, Bornaghi LF, Vullo D, Supuran CT, Poulsen SA (2015) Carbonic anhydrase inhibitors with dual-tail moieties to match the hydrophobic and hydrophilic halves of the carbonic anhydrase active site. J Med Chem 58:1494–1501
Tumosienė I, Jonuškienė I, Kantminienė K, Beresnevičius Z (2012) The synthesis of azole derivatives from 3-[(4-methylphenyl)amino]propanehydrazide and its N’-phenylcarbamoyl derivatives, and their antibacterial activity. Monatsh Chem 143:1441–1450
Tumosienė I, Beresnevičius Z (2009) Synthesis of azoles from 3,3’-[(4-alkoxyphenyl)imino]bis(propanoic acid hydrazides). Monatsh Chem 140:1523–1528
Tumosiene I, Mikulskiene G, Kantminiene K, Beresnevicius Z (2011) Synthesis and structure of 3,3’-[(4-alkoxyphenyl)imino] bis(N’-phthaloyl- or N’-benzylidenepropanohydrazide) derivatives. Chemija 22:65–72
Vaickelioniene R, Mickevicius V, Mikulskiene G (2013) Synthesis and characterization of 4-substituted 1-(4-halogenophenyl)pyrrolidin-2-ones with azole and azine moieties. Heterocycles 87:1059–1074
Winnicka K, Tomasiak M, Bielawska A (2005) Piracetam - an old drug with novel properties? Doxapram. Acta Pol Pharm 62:405–409
Winum JY, Colinas PA, Supuran CT (2013) Glycosidic carbonic anhydrase IX inhibitors: a sweet approach against cancer. Bioorg Med Chem 21:1419–1426
Acknowledgments
This research was funded by the grant (No. TAP LLT-1/2016) from the Research Council of Lithuania.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Rutkauskas, K., Zubrienė, A., Tumosienė, I. et al. Benzenesulfonamides bearing pyrrolidinone moiety as inhibitors of carbonic anhydrase IX: synthesis and binding studies. Med Chem Res 26, 235–246 (2017). https://doi.org/10.1007/s00044-016-1741-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1741-5