Abstract
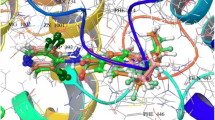
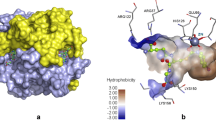
P-gp transporter regulates key ADME of drugs in MDR condition. In the present work, a pharmacophore-based 3D-QSAR model was generated for a series of galloyl benzamides analogs possessing P-gp inhibitory activity. Developed pharmacophore model contains two hydrogen-bond acceptors (A), one hydrophobic (H), one hydrogen-bond donor (D) and two aromatic rings (R). These are crucial molecular fingerprints which predict binding efficacy of high-affinity and low-affinity ligands to the P-gp efflux pump. These pharmacophoric features point toward key structural requirements of galloyl benzamides for potent P-gp inhibition. Furthermore, a biological correlation 3D-QSAR variants and functional fingerprints of P-gp responsible for the receptor binding were observed. Alignment of the developed model with P-gp crystal structure indicated importance of A2 and A4 H-bond acceptor sites, which are involved in the important interactions with Glu530 and His690 residues of the active site. Excellent statistical results of QSAR model such as good correlation coefficient (r 2 > 0.95), higher F value (F > 205) and excellent predictive power (Q 2 > 0.6) with low standard deviation (SD < 0.2) strongly suggest that the developed model is good for the future prediction of P-gp inhibitory activity of new galloyl benzamide analogs.




Similar content being viewed by others
Abbreviations
- 3D-QSAR:
-
3-Dimensional quantitative structure–activity relationship
- P-gp:
-
Permeability-glycoprotein
- PLS:
-
Partial least square
- RMSD:
-
Relative mean square deviation
- RMSE:
-
Root-mean-square error
- SD:
-
Standard deviation
- r 2 :
-
Correlation coefficient
- Q 2 :
-
Cross-validated correlation coefficient
- LOO:
-
Leave-one-out
- ADME:
-
Absorption, distribution, metabolism and excretion
References
Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G (2009) Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323(5922):1718–1722
Bharate JB, Singh S, Wani A, Sharma S, Joshi P, Khan IA, Kumar A, Vishwakarma RA, Bharate SB (2015) Discovery of 4-acetyl-3-(4 fluorophenyl)-1-(p-tolyl)-5-methylpyrrole as a dual inhibitor of human P-glycoprotein and Staphylococcus aureus Nor A efflux pump. Org Biomol Chem 13(19):5424–5431
Chen L, Li Y, Yu H, Zhang L, Hou T (2012) Computational models for predicting substrates or inhibitors of P-glycoprotein. Drug Discov Today 17(7–8):343–351
de Cerqueira Lima P, Golbraikh A, Oloff S, Xiao Y, Tropsha A (2006) Combinatorial QSAR modeling of P-glycoprotein substrates. J Chem Inf Model 46(3):1245–1254
Ferreira RJ, Ferreira M-JU, dos Santos DJVA (2012) Insights on P-glycoprotein’s efflux mechanism obtained by molecular dynamics simulations. J Chem Theory Comput 8(6):1853–1864
Ferreira RJ, Ferreira M-JU, dos Santos DJVA (2013) Molecular docking characterizes substrate-binding sites and efflux modulation mechanisms within P-glycoprotein. J Chem Inf Model 53(7):1747–1760
Fox E, Bates SE (2007) Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor. Expert Rev Anticancer Ther 7(4):447–459
Jin MS, Oldham ML, Zhang Q , Chen J (2012) Crystal structure of the multidrug transporter P-glycoprotein from C. elegans. Nature 490(7421):566–569
Klepsch F, Vasanthanathan P, Ecker GF (2014) Ligand and structure-based classification models for prediction of P-glycoprotein inhibitors. J Chem Inf Model 54(1):218–229
Li D, Chen L, Li Y, Tian S, Sun H, Hou T (2014) ADMET evaluation in drug discovery. 13. Development of in silico prediction models for P-glycoprotein substrates. Mol Pharm 11(3):716–726
Lucas X, Simon S, Schubert R, Günther S (2013) Discovery of the inhibitory effect of a phosphatidylinositol derivative on P-glycoprotein by virtual screening followed by in vitro cellular studies. PLoS ONE 8(4):e60679
Malik R, Sangwan A, Saihgal R, Jindal DP, Piplani P (2007) Towards better brain management: nootropics. Curr Med Chem 14(2):123–131
Pauli-Magnus C, von Richter O, Burk O, Ziegler A, Mettang T, Eichelbaum M, Fromm MF (2000) Characterization of the major metabolites of verapamil as substrates and inhibitors of P-glycoprotein. J Pharmacol Exp Ther 293(2):376–382
Pellicani RZ, Stefanachi A, Niso M, Carotti A, Leonetti F, Nicolotti O, Perrone R, Berardi F, Cellamare S, Colabufo NA (2012) Potent galloyl-based selective modulators targeting multidrug resistance associated protein 1 and P-glycoprotein. J Med Chem 55(1):424–436
Piplani P, Malik R, Kaur B, Kaplish A (2012) Synthesis and pharmacological evaluation of some new naphthol derived aryloxy derivatives as cognition enhancers. Med Chem Res 21(8):1771–1779
Shintre CA, Pike AC, Li Q, Kim JI, Barr AJ, Goubin S, Shrestha L, Yang J, Berridge G, Ross J, Stansfeld PJ, Sansom MS, Edwards AM, Bountra C, Marsden BD, von Delft F, Bullock AN, Gileadi O, Burgess-Brown NA, Carpenter EP (2013) Structures of ABCB10, a human ATP-binding cassette transporter in apo- and nucleotide-bound states. Proc Natl Acad Sci USA 110(24):9710–9715
Singh B, Kumar A, Joshi P, Guru SK, Kumar S, Wani ZA, Mahajan G, Hussain A, Qazi AK, Kumar A, Bharate SS, Gupta BD, Sharma PR, Hamid A, Saxena AK, Mondhe DM, Bhushan S, Bharate SB, Vishwakarma RA (2015) Colchicine derivatives with potent anticancer activity and reduced P-glycoprotein induction liability. Org Biomol Chem 13(20):5674–5689
Stokvis E, Rosing H, Causon RC, Schellens JH, Beijnen JH (2004) Quantitative analysis of the P-glycoprotein inhibitor Elacridar (GF120918) in human and dog plasma using liquid chromatography with tandem mass spectrometric detection. J Mass Spectrom 39(10):1122–1130
Tan W, Mei H, Chao L, Liu T, Pan X, Shu M, Yang L (2013) Combined QSAR and molecule docking studies on predicting P-glycoprotein inhibitors. J Comput Aided Mol Des 27(12):1067–1073
Acknowledgments
Authors are thankful to Schrodinger Inc., USA, and Central University of Rajasthan for providing licensed Schrodinger molecular modeling software.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no financial/commercial conflict of interest.
Rights and permissions
About this article
Cite this article
Srivastava, S., Choudhary, B.S., Sharma, M. et al. Pharmacophore modeling and 3D-QSAR studies of galloyl benzamides as potent P-gp inhibitors. Med Chem Res 25, 1140–1147 (2016). https://doi.org/10.1007/s00044-016-1556-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1556-4