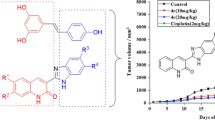
Abstract
A novel class of resveratrol-oxadiazole hybrid compounds was synthesized to screen for their in vitro antiproliferative activity against three human cancer cell lines. All the compounds showed superior antiproliferative activity than the reference compound resveratrol. The most promising active compounds in this series were 1g, 2g, 1c, 2c, 2i and 1a (GI50 < 0.1 µM), endowed with excellent antiproliferative activity. Thus, we believe that resveratrol-oxadiazole hybrid compounds may possibly be used as a good leads for the development of new antiproliferative agents. Structures of newly synthesized compounds were confirmed by NMR and IR spectral data.





Similar content being viewed by others
References
Arbuzov BA (1964) Pure Appl Chem 9:315–370
Bazzo KO, Souto AA, Lopes TG, Zanin RF, Gomez MV, Souza AH, Campos MM (2013) Evidence for the analgesic activity of resveratrol in acute models of nociception in mice. J Nat Prod 76:13–21
Belluti F, Fontana G, Bo LD, Carenini N, Giommarelli C, Zunino F (2010) Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: identification of novel proapoptotic agents. Bioorg Med Chem 18:3543–3550
Burns J, Gardner PT, O’Neil J, Crawford S, Morecroft I, McPhail DB, Lister C, Matthews D, MacLean MR, Lean MEJ, Duthie GG, Crozier A (2000) Relationship among antioxidant activity, vasodilation capacity, and phenolic content of red wines. J Agric Food Chem 48:220–230
Böhm HJ, Banner D, Bendels S, Kansy M, Kuhn B, Müller K, Sander UO, Stahl M (2004) Fluor Med Chem Chembiochem 5:637–643
Cecchinato V, Chiaramonte R, Nizzardo M, Cristofaro B, Basile A, Sherbet GV, Comi P (2007) Resveratrol-induced apoptosis in human T-cell acute lymphoblastic leukaemia MOLT-4 cells. Biochem Pharmacol 74:1568–1574
Charoenrungruang S, Chanvorachote P, Sritularak B, Pongrakhananon V (2014) Gigantol, a bibenzyl from dendrobium draconis, inhibits the migratory behavior of non-small cell lung cancer cells. J Nat Prod 77:1359–1366
Cheung FWK, Leung AWN, Liu WK, Che CT (2014) Tyrosinase inhibitory activity of a glucosylated hydroxystilbene in mouse melan-a melanocytes. J Nat Prod 77:1270–1274
Dörrie J, Gerauer H, Wachter Y, Zunino SJ (2001) Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res 61:4731–4739
Hartung AM, Beutler JA, Navarro HA, Wiemer DF, Neighbors JD (2014) Stilbenes as κ-selective, non-nitrogenous opioid receptor antagonists. J Nat Prod 77:311–319
Houillé B, Papon N, Boudesocque L, Bourdeaud E, Besseau S, Courdavault V, Gueiffier CE, Delanoue G, Guérin L, Bouchara JP, Clastre M, Guivarc’h NG, Guillard J, Lanoue A (2014) Antifungal activity of resveratrol derivatives against candida species. J Nat Prod 77:1658–1662
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Jazirehi AR, Bonavida B (2004) Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin’s lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther 3:71–84
Kamal A, Shaik AB, Polepalli S, Kumar GB, Reddy VS, Mahesh R, Garimella S, Jain N (2015) Synthesis of arylpyrazole linked benzimidazole conjugates as potential microtubule disruptors. Bioorg Med Chem 23:1082–1095
Kim JS, Kang CG, Kim SH, Lee EO (2014) Rhapontigenin suppresses cell migration and invasion by inhibiting the PI3 K-dependent Rac1 signalling pathway in MDA-MB-231 human breast cancer cells. J Nat Prod 77:1135–1139
Koparır M, Çetin A, Cansız A (2005) 5-Furan-2yl[1,3,4]oxadiazole-2-thiol, 5-furan-2yl-4H[1,2,4]triazole-3-thiol and their thiol-thione tautomerism. Molecules 10:475–480
Kumar D, Raj KK, Malhotra SV, Rawat DS (2014) Synthesis and anticancer activity evaluation of resveratrol–chalcone conjugates. Med Chem Commun 5:528–535
Lee I, Choe YS, Choi JY, Lee KH, Kim BT (2012) Synthesis and evaluation of 18F-labeled styryltriazole and resveratrol derivatives for β-amyloid plaque imaging. J Med Chem 55:883–892
Li Y, Liu J, Zhang H, Yang X, Liu Z (2006) Stereoselective synthesis and fungicidal activities of (E)-α-(methoxyimino)-benzeneacetate derivatives containing 1,3,4-oxadiazole ring. Bioorg Med Chem Lett 16:2278–2282
Li H, Wu WKK, Zheng Z, Che CT, Yu L, Li ZJ, Wu YC, Cheng KW, Yu J, Cho CH, Mingfu W (2009) 2,3′,4,4′,5′-pentamethoxy-trans-stilbene, a resveratrol derivative, is a potent inducer of apoptosis in colon cancer cells via targeting microtubules. Biochem Pharmacol 78:1224–1232
Mgbonyebi O, Russo J, Russo I (1998) Antiproliferative effect of synthetic resveratrol on human breast epithelial cells. Int J Oncol 12:865–869
Murty MSR, Raju P, Nath LR, Anto RJ (2014) Synthesis of salicylic acid-based 1,3,4-oxadiazole derivatives coupled with chiral oxazolidinones: novel hybrid heterocycles as antitumor agents. Lett Drug Des Discov 11:1133–1142
Oliveira CS, Lira BF, Filho JMB, Lorenzo JGF, Filho PFA (2012) Synthetic approaches and pharmacological activity of 1,3,4-oxadiazoles: a review of the literature from 2000–2012. Molecules 17:10192–10231
Ozturk S, Akkurt M, Cansiz A, Cetin A, Sekerci M, Heinemann FW (2004) 5-(furan-2-yl)-1,3,4-oxadiazole-2(3H)-thione. Acta Cryst E E 60:O322
Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM (1995) The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta 235:207–219
Pan MH, Lin CL, Tsai JH, Ho CT, Chen WJ (2010) 3,5,3′,4′,5′-Pentamethoxystilbene (MR-5), a synthetically methoxylated analogue of resveratrol, inhibits growth and induces G1 cell cycle arrest of human breast carcinoma MCF-7 cells. J Agric Food Chem 58:226–234
Park BH, Lee HJ, Leem YR (2011) Total synthesis of chiricanine A, arahypin-1, trans-arachidin-2, trans-arachidin-3 and arahypin-5 from peanut seeds. J Nat Prod 74:644–649
Paul S, Mizuno CS, Lee HJ, Zheng X, Chajkowisk S, Rimoldi JM, Conney A, Suh N, Rimando AM (2010) In vitro and In vivo studies on stilbene analogs as potential treatment agents for colon cancer. Eur J Med Chem 45:3702–3708
Reddy MA, Jain N, Yada D, Kishore C, Vangala JR, Surendra PR, Addlagatta A, Kalivendi SV, Sreedhar B (2011) Design and synthesis of resveratrol-based nitrovinylstilbenes as antimitotic agents. J Med Chem 54:6751–6760
Rimando AM, Cuendet M, Desmarchelier C, Mehta RG, Pezzuto JM, Duke SO (2002) Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J Agric Food Chem 50:3453–3457
Roberti M, Pizzirani D, Simoni D, Rondanin R, Baruchello R, Bonora C, Buscemi F, Grimaudo S, Tolomeo M (2003) Synthesis and biological evaluation of resveratrol and analogues as apoptosis-inducing agents. J Med Chem 46:3546–3554
Ruan BF, Lu X, Tang JF, Wei Y, Wang XL, Zhang YB, Wang LS, Zhu HL (2011) Synthesis, biological evaluation, and molecular docking studies of resveratrol derivatives possessing chalcone moiety as potential antitubulin agents. Bioorg Med Chem 19:2688–2695
Savouret JF, Quesne M (2002) Resveratrol and cancer: a review. Biomed Pharmacother 56:84–97
Shen W, Mao J, Sun J, Sun M, Zhang C (2013) Synthesis and biological evaluation of resveratrol–coumarin hybrid compounds as potential antitumor agents. Med Chem Res 22:1630–1640
Udenigwe CC, Ramprasath VR, Aluko RE, Jones PJH (2008) Potential of resveratrol in anticancer and antiinflammatory therapy. Nutr Rev 66:445–454
Wadsworth WS, Emmons WD (1961) The utility of phosphonate carbanions in olefin synthesis. J Am Chem Soc 83:1733–1738
Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK (2004) High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos 32:1377–1382
Yu J, Gaunt MJ, Spencer JB (2002) Convenient preparation of trans-arylalkenes via palladium(II)-catalyzed isomerization of cis- arylalkenes. J Org Chem 67:4627–4629
Acknowledgments
The authors are thankful to the Director, Indian Institute of Chemical Technology, Hyderabad, for the encouragement, RP thankful to CSIR, New Delhi, India for the award of research fellowships. We thank CSIR for financial support under the 12th Five Year plan projects ‘‘Affordable Cancer Therapeutics (ACT)’’ (CSC 0301) and ‘‘Small Molecules in Lead Exploration (SMiLE)’’ (CSC0111).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Murty, M.S.R., Penthala, R., Polepalli, S. et al. Synthesis and biological evaluation of novel resveratrol-oxadiazole hybrid heterocycles as potential antiproliferative agents. Med Chem Res 25, 627–643 (2016). https://doi.org/10.1007/s00044-016-1514-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1514-1