Abstract
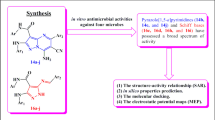
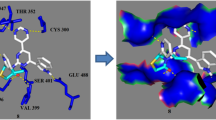
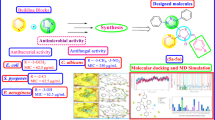
A series of amide-linked 1,4-disubstituted 1,2,3-bistriazoles was tested for antimicrobial activity against Gram-positive bacteria Staphylococcus aureus and Bacillus subtilis, Gram-negative bacteria Escherichia coli and two fungal strains Aspergillus niger and Candida albicans. The antimicrobial evaluation data indicated that most of the compounds exhibited potential activity. To describe activity on the structural basis, QSAR studies were performed and statistically significant models were developed. Further, binding interactions of two active compounds 17 and 10 to active sites of E. coli topoisomerase II DNA gyrase B and C. albicans lanosterol 14α-demethylase (1.14.13.70) (CYPLI) (cytochrome P450 51) enzymes, respectively, were also examined.












Similar content being viewed by others
References
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
AutoDock Tools (version 1.5.6 rc2), Stefano Forte. Molecular Graphics Laboratory, Department of Molecular Biology, The Scripps Research Institute, (1999–2010) http://mgltools.scripps.edu
Bakunov SA, Bakunova SM, Wenzler T, Ghebru M, Werbovetz KA, Brun R, Tidwell RR (2010) Synthesis and antiprotozoal activity of cationic 1,4-diphenyl-1H-1,2,3-triazoles. J Med Chem 53(1):254–272
Benkert P, Biasini M, Schwede T (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27(3):343–350
Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Cassarino TG, Bertoni M, Bordoli L, Schwede T (2014) SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42:252–258
Cao X, Sun Z, Cao Y, Wang R, Cai T, Chu W, Hu W, Yang Y (2014) Design, synthesis, and structure-activity relationship studies of novel fused heterocycles-linked triazoles with good activity and water solubility. J Med Chem 57(9):3687–3706
Cappucino JG, Sherman N (1999) Microbiology—a laboratory manual, 4th edn. Addison Wesley Longman Inc., Harlow, p 263
Discovery Studio v4.0 client, Accelrys Software Inc. (2005–2014)
Dudek AZ, Arodzb T, Gálvez J (2006) Computational methods in developing quantitative structure-activity relationships (QSAR): a review. Comb Chem High Throughput Screen 9(3):213–228
Dunbrack RL (2002) Rotamer libraries in 21st century. Curr Opin Struct Biol 12(4):431–440
Furusjo E, Svenson A, Rahmberg M, Andersson M (2006) Chemosphere 63(1):99–108
Genin MJ, Allwine DA, Anderson DJ, Barbachyn MR, Emmert DE, Garmon SA, Graber DR et al (2000) Substituent effects on the antibacterial activity of nitrogen-carbon-linked (azolylphenyl)oxazolidinones with expanded activity against the fastidious gram-negative organisms Haemophilus influenzae and Moraxella catarrhalis. J Med Chem 43(5):953–970
Ghose AK, Crippen GM (1986) Atomic physicochemical parameters for three-dimensional structure-directed quantitative structure-activity relationships. I. Partition coefficients as a measure of hydrophobicity. J Chem Inf Comput Sci 7:565–577
Ghose AK, Crippen GM (1987) Atomic physicochemical parameters for three-dimensional-structure-directed quantitative structure-activity relationships. Modeling dispersive and hydrophobic interactions. J Chem Inf Comput Sci 27(1):21–35
Golbraikh A, Tropsha A (2002) Beware of q2! J Mol Graph Model 20(4):269–276
Gramatica P (2007) Principles of QSAR models validation: internal and external. QSAR Comb Sci 26(5):694–701
Ji H, Zhang W, Zhou Y, Zhang M, Zhu J, Song Y, Lu¨ J, Zhu J (2000) A three-dimensional model of Lanosterol 14α-Demethylase of Candida albicans and its interaction with azole antifungals. J Med Chem 43(13):2493–2505
Kaushik CP, Lal K, Kumar A, Kumar S (2014) Synthesis and biological evaluation of amino acid-linked 1,2,3-bistriazole conjugates as potential antimicrobial agents. Med Chem Res 23(6):2995–3004
Kier LB, Hall LH (1976) Molecular connectivity in chemistry and drug research. Academic Press, New York
Kumar A, Kumar S, Jain S, Kumar P, Goyal R (2013) Study of binding of pyridoacridine alkaloids on Topoisomerase II using in silico tools. Med Chem Res 22(11):5431–5441
Lal K, Kaushik CP, Kumar K, Kumar A, Qazi AK, Hamid A, Jaglan S (2014) One-pot synthesis and cytotoxic evaluation of amide-linked 1,4-disubstituted 1,2,3-bistriazoles. Med Chem Res 23(8):4761–4770
Lauria A, Delisi R, Mingoia F, Terenzi A, Martorana A, Barone G, Almerico AM (2014) 1,2,3-Triazole in heterocyclic compounds, endowed with biological activity, through 1,3-dipolar cycloadditions. Eur J Org Chem 16:3289–3306
Liu S, Cao C, Li Z (1998) Approach to estimation and prediction for normal boiling point (NBP) of alkanes based on a novel molecular distance edge (MDE) Vector, lambda. J Chem Inf Comput Sci 38:387–394
Na YM (2011) Synthesis and activity of novel 1-halogenobenzylindole linked triazole derivatives as antifungal agents. Bull Korean Chem Soc 32(1):307–310
Mavin Sketch 5.10.1 ChemAxon Ltd. (1998–2012) http://www.chemaxon.com
Ostrov DA, Prada JAH, Corsino PE, Finton KA, Le N, Rowe TC (2007) Discovery of novel DNA gyrase inhibitors by high-throughput virtual screening. Antimicrob Agents Chemother 51(10):3688–3698
Patpi SR, Pulipati L, Yogeeswari P, Sriram D, Jain N, Sridhar B, Murthy R, Devi AT et al (2012) Design, synthesis, and structure-activity correlations of novel dibenzo[b, d]furan, dibenzo[b, d]thiophene, and N-methylcarbazole clubbed 1,2,3-triazoles as potent inhibitors of Mycobacterium tuberculosis. J Med Chem 55(8):3911–3922
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF chimera a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612
Puzyn T, Leszczynski J, Cronin MTD (2010) Recent advances in QSAR studies: methods and applications. Cronin MTD quantitative structure–activity relationships (QSAR)—applications and methodology, 8th edn. Springer, New York, pp 3–11
PyMOL(TM) Molecular Graphics System, Version 0.99rc6, Copyright © 2006 DeLano Scientific LLC
Singh P, Raj R, Kumar V, Mahajan MP, Bedi PM, Kaur T, Saxena AK (2012) 1,2,3-Triazole tethered β-lactam-chalcone bifunctional hybrids: synthesis and anticancer evaluation. Eur J Med Chem 47(1):594–600
Stanton DT, Jurs PC (1990) Development and use of charged partial surface area structural descriptors in computer assisted quantitative structure property relationship studies. Anal Chem 62(21):2323–2329
Surineni G, Yogeeswari P, Sriram D, Kantevari S (2015) Rational design, synthesis and evaluation of novel-substituted 1,2,3-triazolylmethyl carbazoles as potent inhibitors of Mycobacterium tuberculosis. Med Chem Res 24(3):1298–1309
Todeschini R, Gramatica P (1997) The Whim theory: new 3D molecular descriptors for QSAR in environmental modeling. SAR and QSAR Environ Res 7:89–115
Todeschini R, Gramatica P (1998) New 3D molecular descriptors: the WHIM theory and QAR applications. Persepect Drug Discov 9:355–380
Todeschini R, Lasagni M, Marengo E (1994) New molecular descriptors for 2D and 3D structures. J Chemom 8:263–272
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 31(2):455–461
Wang J, Wang W, Kollman PA, Case DA (2006) Automatic atom type and bond type perception in molecular mechanical calculations. J Mol Graph Model 25(2):247–260
Whiting M, Tripp JC, Lin YC, Lindstorm W, Olson AJ, Elder JH, Sharpless KB, Fokin VV (2006) Rapid discovery and structure activity profiling of novel inhibitors of human immunodeficiency virus type 1 protease enabled by the copper(I)-catalyzed synthesis of 1,2,3-triazoles and their further functionalization. J Med Chem 49(26):7697–7710
Yap CW (2011) PaDEL-Descriptor: an open source software to calculate molecular descriptors and fingerprints. J Comput Chem 32(7): 1466–1474
Acknowledgments
Authors are highly thankful for financial assistance from University Grants Commission, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lal, K., Kaushik, C.P. & Kumar, A. Antimicrobial evaluation, QSAR and docking studies of amide-linked 1,4-disubstituted 1,2,3-bistriazoles. Med Chem Res 24, 3258–3271 (2015). https://doi.org/10.1007/s00044-015-1378-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-015-1378-9