Abstract
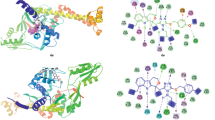
The present paper elicits an unprecedented synthesis of a novel series of 1,2,3-triazole compounds using geraniol as the precursor via 1,3-dipolar cycloaddition using click chemistry strategy. All the synthesized compounds were screened to evaluate their microbicidal and antioxidant potentials. The antibacterial activity of all the new compounds was assessed by the micro-broth dilution technique against a panel of Gram-positive Staphylococcus aureus (MTCC 3160), Bacillus cereus (MTCC 430), and Gram-negative Escherichia coli (MTCC 1610) and Pseudomonas aeruginosa (MTCC 2295). Furthermore, evaluation of their antioxidative behavior manifested the remarkable free radical scavenging activity using DPPH assay. Also, the docking study of the compounds was performed on complex structure of E.coli FabH (1HNJ. pdb) using VLife MDS 3.5 software. Significant dock score of 5g (−80.121 kcal/mol) in comparison with the standard ciprofloxacin (−88.263 kcal/mol) has been figured out upon molecular docking.








Similar content being viewed by others
References
Almasirad A, Tabatabai SA, Faizi M, Kebriaezadeh A, Mehrabi N, Dalvandi F, Shafiee A (2004) Synthesis and anticonvulsant activity of new 2-substituted-5- [2-(2-fluorophenoxy)phenyl]-1,3,4-oxadiazoles and 1,2,4-triazoles. Bioorg Med Chem Lett 14:6057–6059
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils—a review. Food Chem Toxicol 46:446–475
Bandyopadhyay P, Sathe M, Ponmariappan S, Sharma A, Sharma P, Srivastava AK, Kaushik MP (2011) Exploration of in vitro time point quantitative evaluation of newly synthesized benzimidazole and benzothiazole derivatives as potential antibacterial agents. Bioorg Med Chem Lett 21:7306–7309
Barbuceanu SF, Saramet G, Almajan GL, Draghici C, Barbuceanu F, Bancescu G (2012) New heterocyclic compounds from 1,2,4-triazole and 1,3,4-thiadiazole class bearing diphenylsulfone moieties: synthesis, characterization and antimicrobial activity evaluation. Eur J Med Chem 49:417–423
Clinical and Laboratory Standard Institute (2007) Performance standards for antimicrobial susceptibility testing; seventeenth informational supplement: CLSI document M100-S17. Clinical and Laboratory Standard Institute, Wayne, pp 1987–1988
De Clercq E (2004) Antiviral drugs in current clinical use. J Clin Virol 30:115–133
Delgado JN, Remers WA (1998) Text book of organic, medicinal and pharmaceutical chemistry, tenth edn. J.B. Lippincott Company, Philadelphia
Demirbas N, Ugurluoglu R (2002) Synthesis of 3-alkyl(Aryl)-4-alkylidenamino-4,5-dihydro-1H-1,2,4-triazol-5-ones and 3-alkyl-4-alkylamino-4,5-dihydro-1H-1,2,4-triazol-5-ones as antitumor agents. Bioorg Med Chem 10:3717–3723
Ezabadi IR, Camoutsis C, Zoumpoulakis P, Geronikaki A, Sokovic M, Glamocilijad J, Ciric A (2008) Ulfonamide-1,2,4-triazole derivatives as antifungal and antibacterial agents: synthesis biological evaluation, lipophilicity, and conformational studies. Bioorg Med Chem 16:1150–1161
Gasteiger J, Marsili M (1980) Iterative partial equalization of orbital electronegativity—a rapid access to atomic charges. Tetrahedron 36:3219–3228
Guzeldemirci NU, Kucukbasmaci O (2010) Synthesis and antimicrobial activity evaluation of new 1,2,4-triazoles and 1,3,4-thiadiazoles bearing imidazo[2,1-b]thiazole moiety. Eur J Med Chem 45:63–68
Halgren TA (1996) Molecular geometries and vibrational frequencies for MMFF94. J Comput Chem 17:553–586
Harini ST, Kumar HV, Rangaswamy J, Naik N (2012) Synthesis, antioxidant and antimicrobial activity of novel vanillin derived piperidin-4-one oxime esters: preponderant role of the phenyl ester substituents on the piperidin-4-one oxime core. Bioorg Med Chem 22:7588–7592
Huisgen R (1963) 1,3-Dipolare Cycloadditionen Rückschau und Ausblick. Angew Chem 75:604–637
Ishikawa T, Mizutani A, Miwa C, Oku Y, Komano N, Takami A, Watanabe T (1997) Cesium fluoride-mediated claisen rearrangements of phenyl propargyl ethers: substituent effects of an ortho-alkoxy group on the benzene ring or modified propargyl residues. Heterocycles 45:2261–2272
Ji D, Lu JR, Lu BW, Xin CW, Mu JB, Li JF, Peng CY, Bao XR (2013) Efficient synthesis and antimicrobial activity of some novel S-β-d-glucosides of 5-aryl-1,2,4-triazole-3-thiones derivatives. Bioorg Med Chem Lett 23:1997–2000
Joshi MC, Joshi P, Rawat DS (2006) Microwave assisted synthesis of symmetrically and asymmetrically substituted acyclic enediynes. Arkivoc 16:65–74
Khalil NSAM (2010) Efficient synthesis of novel 1,2,4-triazole fused acyclic and 21-28 membered macrocyclic and/or lariat macrocyclic oxaazathia crown compounds with potential antimicrobial activity. Eur J Med Chem 45:5265–5277
Kim SH, Bae HC, Park EJ, Lee CR, Kim BJ, Lee S, Park HH, Kim SJ, So I, Kim TW, Jeon JH (2011) Geraniol inhibits prostate cancer growth by targeting cell cycle and apoptosis pathways. Biochem Biophys Res Commun 407:129–134
Kolb HC, Finn MG, Sharpless KB (2001) Click-Chemie: diverse chemische Funktionalität mit einer Handvoll guter Reaktionen. Angew Chem 113:2056–2075
Koparir M, Orek C, Parlak AE, Soylemez A, Koparir P, Karatepe M, Dastan SD (2013) Synthesis and biological activities of some novel aminomethyl derivatives of 4-substituted-5-(2-thienyl)-2,4-dihydro-3H-1,2,4-triazole-3-thiones. Eur J Med Chem 63:340–346
Kucukguzel I, Kucukguzel SG, Rollas S, Kiraz M (2001) Some 3-Thioxo/alkylthio-1,2,4-triazoles with a substituted thiourea moiety as possible antimycobacterials. Bioorg Med Chem 11:1703–1707
Kumar A, Sharma P, Gurram VK, Rane N (2006) Studies on synthesis and evaluation of quantitative structure–activity relationship of 10-methyl-6-oxo-5-arylazo-6,7-dihydro-5H-[1,3]azaphospholo[1,5-d][1,4] benzodiazepin-2-phospha-3-ethoxycarbonyl-1-phosphorus dichlorides. Bioorg Med Chem Lett 16:2484–2491
Narsinghani T, Sharma MC, Bhargav S (2013) Synthesis, docking studies and antioxidant activity of some chalcone and aurone derivatives. Med Chem Res 22:4059–4068
Pal M, Parasuraman K, Yeleswarapu KR (2003) Palladium-catalyzed cleavage of O/N-propargyl protecting groups in aqueous media under a copper-free condition. Org Lett 5:349–352
Qiu X, Janson CA, Smith WW, Head M, Lonsdale J, Konstantinidis AK (2001) Refined structures of β-ketoacyl-acyl carrier protein synthase III. J Mol Biol 307:341–356
Radhika C, Venkatesham A, Sarangapani M (2012) Synthesis and antidepressant activity of di substituted-5-aryl-1,2,4-triazoles. Med Chem Res 21:3509–3513
Reddy MVR, Billa VK, Pallela VR, Mallireddigari MR, Boominathan R, Gabriel JL, Reddy EP (2008) Design, synthesis, and biological evaluation of 1-(4-sulfamylphenyl)-3-trifluoromethyl-5-indolyl pyrazolines as cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) inhibitors. Bioorg Med Chem 16:3907–3916
Sharma P, Rane N, Gurram VK (2004) Synthesis and QSAR studies of pyrimido[4,5-d]pyrimidine-2,5-dione derivatives as potential antimicrobial agents. Bioorg Med Chem Lett 14:4185–4190
Sharma P, Kumar A, Rane N, Gurram V (2005a) Hetero Diels-Alder reaction: a novel strategy to regioselective synthesis of pyrimido[4,5-d]pyrimidine analogues from Biginelli derivative. Tetrahedron 61:4237–4248
Sharma P, Kumar A, Sharma S, Rane N (2005b) Studies on synthesis and evaluation of quantitative structure–activity relationship of 5-[(30-chloro-40,40-disubstituted-2-oxoazetidinyl)(N-nitro)amino]-6-hydroxy-3-alkyl/aryl[1,3]-azaphospholo[1,5-a]pyridin-1-yl-phosphorus dichlorides. Bioorg Med Chem Lett 5:937–943
Sharma P, Kumar A, Upadhyay S, Sahu V, Singh J (2008) Synthesis and QSAR modeling of 2-acetyl-2-ethoxycarbonyl-1-[4(4′-arylazo)-phenyl]-N, N-dimethylaminophenyl aziridines as potential antibacterial agents. Eur J Med Chem 16:251–259
Sharma P, Kumar A, Sahu V (2010) Theoretical evaluation of global and local electrophilicity patterns to characterize hetero-Diels-Alder cycloaddition of three-membered 2H-azirine ring system. J Phys Chem A 114:1032–1038
Siddiqui AA, Mishra R, Shaharyar M, Husain A, Rashid M, Pal P (2011) Triazole incorporated pyridazinones as a new class of antihypertensive agents: design, synthesis and in vivo screening. Bioorg Med Chem 21:1023–1026
Singh P, Kaur S, Kumar V, Bedi PMS, Mahajan MP, Sehar I, Pal HC, Saxena AK (2011) Synthesis and in vitro cytotoxic evaluation of N-alkylbromo and N-alkylphthalimido-isatins. Bioorg Med Chem Lett 21:3017–3020
Sudheendran K, Schmidt D, Frey W, Conrad J, Beifuss U (2014) Facile synthesis of 3,5-diaryl-1,2,4-triazoles via copper-catalyzed domino nucleophilic substitution/oxidative cyclization using amidines or imidates as substrates. Tetrahedron 70:1635–1645
Thomas KD, Adhikari AV, Shetty NS (2010) Design, synthesis and antimicrobial activities of some new quinoline derivatives carrying 1,2,3-triazole moiety. Eur J Med Chem 45:3803–3810
Tiwari M, Kakkar P (2009) Plant derived antioxidants—geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol In Vitro 23:295–301
Trakossas S, Argyropoulou EC, Litina DJH (2011) Synthesis of modified triazole nucleosides possessing one or two base moieties via a click chemistry approach. Tetrahedron Lett 52:1673–1676
Vijesh AM, Isloor AM, Shetty P, Sundershan S, Fun HK (2013) New pyrazole derivatives containing 1,2,4-triazoles and benzoxazoles as potent antimicrobial and analgesic agents. Eur J Med Chem 62:410–415
VLife MDS 3.5 Molecular design suite, VLife Sciences Technologies, Pune (2008) www.vlifesciences.com
Zhang F, Wen Q, Wang SF, Karim BS, Yang YS, Liu JJ, Zhang WM, Zhu HL (2014) Design, synthesis and antibacterial activities of 5-(pyrazin-2-yl)-4H-1,2,4-triazole-3-thiol derivatives containing Schiff base formation as FabH inhibitory. Bioorg Med Chem Lett 24:90–95
Zoumpoulakis P, Camoutsis C, Pairas G, Sokovic M, Glamoclija J, Potamitis C, Pitsas A (2012) Synthesis of novel sulfonamide-1, 2, 4-triazoles, 1, 3, 4-thiadiazoles and 1, 3, 4-oxadiazoles, as potential antibacterial and antifungal agents: biological evaluation and conformational analysis studies. Bioorg Med Chem 20:1569–1583
Acknowledgments
We are thankful to Defence Research and Development Organization (DRDO), New Delhi, for providing financial support for the research project (ERIP/ER/1103024/M/01/1476). We thank VLife Science Technologies Pvt. Ltd for providing the software for the study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dubey, N., Sharma, M.C., Kumar, A. et al. A click chemistry strategy to synthesize geraniol-coupled 1,4-disubstituted 1,2,3-triazoles and exploration of their microbicidal and antioxidant potential with molecular docking profile. Med Chem Res 24, 2717–2731 (2015). https://doi.org/10.1007/s00044-015-1329-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-015-1329-5