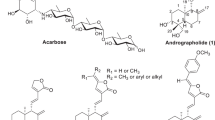
Abstract
A series of oxygen and nitrogen derivatives of 29-norcycloartane triterpenoids have been synthesized on the basis of cyclomusalenone isolated from Musa balbisiana Colla. The structures of new compounds have been established by an NMR spectroscopy analysis, and inhibitory activity against α-glucosidase was evaluated. Among tested compounds, 2,3-isoxazolocyclomusalenone is discovered as a new potent α-glucosidase inhibitor with IC50 value 20.4 μM being 30- and 20-fold more active than initial cyclomusalenone and marketed drug acarbose.
Graphical Abstract



Similar content being viewed by others
References
Akihisa T, Shimizu N, Tamura T, Matsumoto T (1986) (24S)-14a,24-Dimethyl-9b,19-Cyclo-5a-Cholest-25-en-3β-ol: a new sterol and other sterols in Musa sapientum. Lipids 21:494–497
Akihisa T, Kimura Y, Kokke WMC, Takase S, Yasukawa K, Jin-Nai A, Tamura T (1997) 4-Epicycloeucalenone and 4-epicyclomusalenone: two 3-oxo-28- norcycloartanes from the fruit peel of Musa sapientum L. Chem Pharm Bull 45:744–746
Andrade-Cetto A, Heinrich M (2005) Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J Ethnopharmacol 99:325–348
Benalla W, Bellahcen S, Bnouham M (2010) Antidiabetic medicinal plants as a source of alpha-glucosidase inhibitors. Curr Diabetes Rev 6:247–254
Bischoff H (1994) Pharmacology of alpha-glucosidase inhibition. Eur J Clin Invest 24:3–10
Chu T, Zhu L, Li J, Qin R, Liu C, Chen Y, Yang G (2012) Synthesis and structure elucidation of five new conjugates of oleanolic acid derivatives and chalcones using 1D and 2D NMR spectroscopy. MRC Letter 50:236–241
Davis S (2012) Oral hypoglycaemic drugs for the treatment of type 2 diabetes mellitus. SA Pharmaceutical J 79:22–26
De Melo EB, Da Silveria GA, Carvalho I (2006) α- and β-Glucosidase inhibitors: chemical structure and biological activity. Tetrahedron 62:10277–10302
Dioum EM, Osborne JK, Goetsch S, Russell J, Schneider JW, Cobb MH (2011) A small molecule differentiation inducer increases insulin production by pancreatic beta-cells. Proc Natl Acad Sci USA 108:20713–20718
Genet C, Strehle A, Scmidt C, Boudjelal G, Lobstein A, Schoonjans K, Souchet M, Auwerx J, Saladin R, Wagner A (2010) Structure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: potential impact in diabetes. J Med Chem 53:178–190
Guo ZH, Huang J, Wan GS, Huo XL, Gao HY (2013) New inhibitors of α-glucosidase in Salacia hainanensis Chun et how. J Nat Med 67:844–849
Hung H-V, Qian K, Morris-Natschke SL, Hsu C-S, Lee K-H (2012) Recent discovery of plant-derived anti-diabetic natural products. Nat Prod Rep 29:580–606
Jeong TY, Park BK, Cho JH, Kim YI, Ahn YC, Son CG (2012) A prospective study on the safety of herbal medicines, used alone or with conventional medicines. J Ethnopharmacol 143:884–888
Kashiwada Y, Nishimura K, Kurimoto S, Takaishi Y (2011) New 29-nor-cycloartanes with a 3,4-seco- and a novel 2,3-seco-structure from the leaves of Sinocalycanthus chinensis. Bioorg Med Chem 19:2790–2796
Kim YM, Wang MH, Rhee HI (2004) A novel α-glucosidase inhibitor from pine bark. Carbohydr Res 339:715–717
Knapp FF, Nicholas HJ (1970) The isolation of 31-norcyclolaudenone from Musa sapientum. Steroids 16:329–351
Kuo R-Y, Qian K, Morris-Natschke SL, Lee K-H (2009) Plant-derived triterpenoids and analogues as antitumor and anti-HIV agents. Nat Prod Rep 26:1321–1344
Lee JH, Cuong TD, Kwack SJ, Seok JH, Lee JK, Jeong JY, Woo M-H, Choi JS, Lee HK, Min B-S (2012) Cycloartane-type triterpene glycosides from the rhizomes of Cimicifuga heracleifolia and their anticomplementary activity. Planta Med 78:1391–1394
Li W, Zheng HC, Bukuru J, De Kimpe N (2004) Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol 92:1–21
Li T, Zhang XD, Song YW, Liu JW (2005) A microplate-based screening method for α-glucosidase inhibitors. Nat Prod Res Dev 10:1128–1134
Mukherjee PK, Maiti K, Mukherjee K, Houghton PJ (2006) Leads from Indian medicinal plants with hypoglycemic potentials. J Ethnopharmacol 106:1–28
Omar EA, Kam A, Alqahtani A, Li KM, Razmovski-Naumovski V, Nammi S, Chann K, Roufogalis BD, Li GQ (2010) Herbal medicines and nutraceuticals for diabetic vascular complications: mechanisms of action and bioactive phytochemicals. Curr Pharm Des 16:3776–3807
Pudhom K, Nuanyai T, Matsubara K, Vilaivan T (2012) Antiangiogenic activity of 3,4-seco-cycloartane triterpenes from Thai Gardenia spp. and their semi-synthetic analogs. Bioorg Med Chem Lett 22:512–517
Quisumbing E (1978) Medicinal plants of the Philippines. Bureau of printing, Manila, pp 553–554
Ramírez-Cisneros MA, Rios MY, Déciga-Campos M, Aguilar-Guadarrama AB (2012) Phytochemical study and anti-inflammatory, antidiabetic and free radical scavenger evaluations of Krameria pauciflora methanol extract. Molecules 17:861–872
Rees DA, Alcolado JC (2005) Animal models of diabetes mellitus. Diabet Med 22:359–370
Shen T, Yuan H-Q, Wan W-Z, Wang X-N, Ji M, Lou H-X (2008) Cycloartane-type triterpenoids from the resinous exudates of Commiphora opobalsamum. J Nat Prod 71:81–86
Smirnova IE, Huong DTT, Kazakova OB, Tolstikov GA, Lobov AN, Syponitsky KYu (2012) Ozonolysis of cyclomusalenone and its derivatives. Chem Nat Comp 48:816–820
Sun R, Song H-C, Wang C-R, Shen K-Z, Xu Y-B, Gao Y-X, Chen Y-G, Dong J-Y (2011) Compounds from Kadsura angustifolia with anti-HIV activity. Bioorg Med Chem Lett 21:961–965
Thuong PT, Lee CH, Dao TT, Nguyen PH, Kim WG, Lee SJ, Oh WK (2008) Triterpenoids from the leaves of Diospyros kaki (persimmon) and their inhibitory effects on protein tyrosine phosphatise 1B. J Nat Prod 71:1775–1778
Truong NB, Pham CV, Doan HTM, Nguyen HV, Nguyen CM, Nguyen HT, Zhang H-J, Fong HH, Franzblau SG, Soejarto DD, Chau MV (2011) Antituberculosis cycloartane triterpenoids from Radermachera boniana. J Nat Prod 74:1318–1322
Van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, Rutten GE, van Wheel (2005) Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane: systematic review and meta-analysis. Diabetes Care 28:154–163
Viet DQ, Van Sung T, Thuy NT (2006) Preliminary study on glucose-lowering effect of Musa balbisiana in experimental mice. Vietnamese J Pharmacy 5(361):8–10
Yang J-L, Shi Y-P (2012) Structurally diverse terpenoids from the Rhizomes of Cyperus rotundus L. Planta Med 78:59–64
Zeid A (1998) Chemical and biological study of the leaves of some Musa species. Egypt J Pharm Sci 39:379–398
Zhou JW, Yan JF, Tang XM, Zhang WY, Zhang YX, Chen X, Su XY, Yang DC (1975) Synthesis and preliminary evaluation of antidiabetic activity for β-amino ketone containing isoxazole moiety. Chin J Org Chem 30:582–589
Acknowledgments
This work was partially supported by the Russian Foundation for Basic Research [No.: 10-03-90303]; Vietnam Academy of Science and Technology (VAST) for International projects (2010–2011); Grant of the President of Russian Federation for support of young scientists [MK-5382.2013.3].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smirnova, I.E., Kazakova, O.B., Viet, D.Q. et al. Synthesis and evaluation of 29-norcycloartane triterpenoids as α-glucosidase inhibitors. Med Chem Res 24, 2177–2182 (2015). https://doi.org/10.1007/s00044-014-1292-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1292-6