Abstract
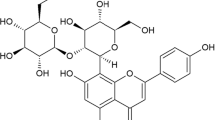
This paper attempts to evaluate the anti-inflammatory potential and the possible mechanism of action of the leaf extracts and isolated compound(s) of Aerva sanguinolenta (Amaranthaceae), traditionally used in ailments related to inflammation. The anti-inflammatory activity of ethanol extract (ASE) was evaluated by acute, subacute and chronic models of inflammation, while a new cerebroside (‘trans’, ASE-1), isolated from the bioactive ASE and characterized spectroscopically, was tested by carrageenan-induced mouse paw oedema and protein exudation model. To understand the underlying mechanism, we measured the release of pro-inflammatory mediators such as nitric oxide (NO) and prostaglandin (PG)E2, along with the cytokines like tumour necrosis factor (TNF)-α, and interleukins(IL)-1β, IL-6 and IL-12 from lipopolysaccharide (LPS)-stimulated peritoneal macrophages. The results revealed that ASE at 400 mg/kg caused significant reduction of rat paw oedema, granuloma and exudative inflammation, while the inhibition of mouse paw oedema and exudative inflammation by ASE-1 (20 mg/kg) was comparable to that of the standard drug indomethacin (10 mg/kg). Interestingly, both ASE and ASE-1 showed significant inhibition of the expressions of iNOS2 and COX-2, and the down-regulation of the expressions of IL-1β, IL-6, IL-12 and TNF-α, in LPS-stimulated macrophages, via the inhibition of COX-2-mediated PGE2 release. Thus, our results validated the traditional use of A. sanguinolenta leaves in inflammation management.







Similar content being viewed by others
References
Adhikari BS, Babu MM, Saklani PL, Rawat GS (2010) Medicinal plants diversity and their conservation status in Wildlife Institute of India (WII) Campus Dehradun. Ethnobot Leaflets 14:46–83
Alam MM, Islam SA, Mohammed Y, Juyena NS, Hasim MA (2005) Comparative efficacy of two medicinal plant extracts and an antibiotic on wound healing. Pakistan J Biol Sci 8:740–743
Alcaraz MJ, Jimenez MJ (1988) Flavonoids as anti-inflammatory agents. Fitoterapia 59:25–38
Betz M, Fox BS (1991) Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol 146:108–113
Cateni F, Zilic J, Falsone G, Hollan F, Frausin F, Scarcia V (2003a) Preliminary biological assay on cerebroside mixture from Euphorbia nicaeensis All. Isolation and structure determination of five glucocerebrosides. Il Farmaco 58:809–817
Cateni F, Zilic J, Falsone G, Scialino G, Banfi E (2003b) New cerebrosides from Euphorbia peplis L.: antimicrobial activity evaluation. Bioorg Med Chem Lett 13:4345–4350
Cateni F, Zilic J, Zacchigna M (2008) Isolation and structure elucidation of cerebrosides from Euphorbia platyphyllos L. Sci Pharm 76:451–469
Chattopadhyay D, Khan MTH (2008) Ethnomedicines and ethnomedicinal phytophores against herpesviruses. Biotechnol Annu Rev 14:297–348
Chattopadhyay D, Arunachalam G, Mandal AB, Sur TK, Mandal SC (2002) Antimicrobial and anti-inflammatory activity of Mallotus peltatus leaf extract. J Ethnopharmacol 82(2–3):229–237
Chattopadhyay D, Mukherjee H, Bag P, Ojha D, Konreddy AK, Dutta S, Haldar P, Chatterjee T, Sharon A, Chakraborti S (2012) Inhibition of NO2, PGE2, TNF-α and iNOS expression by Shorea robusta L.: an ethnomedicine used for antiinflammatory and analgesic activity. Evidence-Based Complement Altern Med 2012, ID: 254849
Colditz IG (1985) Margination and emigration of leucocytes. Surv Synth Pathol Res 4(1):44–68
D’Arcy PF, Haward EM, Muggleton RW, Townsend SB (1960) The anti-inflammatory action of griseofulvin in experimental animals. J Pharm Pharmacol 12:659–665
Das S, Mukherjee H, Ahmed SKM, Haldar PK, Mandal AB, Mahapatra A, Mukherjee PK, Chakraborti S, Chattopadhyay D (2013) Evaluation of an ethnomedicinal combination containing Semecarpus kurzii and Hernandia peltata used for the management of inflammation. Pharm Biol 51(6):677–685
Dewanjee S, Dua TK, Sahu R (2013) Potential anti-inflammatory effect of Leea macrophylla Roxb. leaves: a wild edible plant. Food Chem Toxicol 59:514–520
Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE (2007) Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol 147(2):227–235
Fujiwara N, Kobayashi K (2005) Macrophages in inflammation. Curr Drug Targets Inflamm Allergy 4:281–286
Gaddi A, Cicero AFG, Pedro EJ (2004) Clinical perspectives of anti-inflammatory therapy in the elderly: the lipoxigenase (LOX)/cyclooxigenase (COX) inhibition concept. Arch Gerontol Geriatr 38(3):201–212
Ghosh S, Chattopadhyay D, Mandal A, Kaity S, Samanta A (2013) Bioactivity guided isolation of antiinflammatory, analgesic, and antipyretic constituents from the leaves of Pedilanthus tithymaloides (L.). Med Chem Res 22:4347–4359
Griffon B, Cillard J, Chevanne M, Morel I, Cillard P, Sergent O (1998) Macrophage-induced inhibition of nitric oxide production in primary rat hepatocyte cultures via prostaglandin E2 release. Hepatology 28:1300–1308
Gupta M, Mazumder UK, Gomathi P, Selvan VT (2006) Antiinflammatory evaluation of leaves of Plumeria acuminata. BMC Complement Altern Med 6(1):36. doi:10.1186/1472-6882-6-36
Harbrecht BG, Kim YM, Wirant EA, Simmons RL, Billiar TR (1997) Timing of prostaglandin exposure is critical for the inhibition of LPS- or IFN-gamma-induced macrophage NO synthesis by PGE2. J Leukoc Biol 61(6):712–720
Heller A, Koch T, Schmeck J, Acker VK (1998) Lipid mediators in inflammatory disorders. Drugs 55:487–496
Hibbs JJB, Taintor RR, Vavrin Z, Rachlin EM (1988) Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun 157(1):87–94
Ibrahim B, Sowemimo A, Van Rooyen A, Van de Venter VM (2012) Anti-inflammatory, analgesic and antioxidant activities of Cyathula prostrata (Linn.) Blume (Amaranthaceae). J Ethnopharmacol 141(1):282–289
Ishola IO, Agbaje OE, Narender T, Adeyemi OO, Shukla R (2012) Bioactivity guided isolation of analgesic and anti-inflammatory constituents of Cnestis ferruginea Vahl ex DC (Connaraceae) root. J Ethnopharmacol 142:383–389
Jalalpure SS, Patil MB, Prakash NS, Hemalata K, Manvi FV (2003) Hepatoprotective activity of the fruits of Piper longum Linn. Indian J Pharm Sci 65:363–366
Kabir MH, Hasan N, Rahman MM, Rahman MA, Khan JA, Hoque NT, Bhuiyan MR, Mou SM, Jahan R, Rahmatullah M (2014) A survey of medicinal plants used by the Deb barma clan of the Tripura tribe of Moulvibazar district. Bangladesh. J Ethnobiol Ethnomed 6(10):19–47
Kang SS, Kim JS, Son KH, Kim HP, Chang HW (2001) Cyclooxygenase-2 inhibitory cerebrosides from Phytolaccae Radix. Chem Pharm Bull 49:321–323
Kasama T, Strieter RM, Standiford TJ, Burdick MD, Kunkel SL (1993) Expression and regulation of human neutrophil-derived macrophage inflammatory protein 1α. J Exp Med 178(1):63–72
Klingemann HG, Tsoi MS, Storb R (1986) Inhibition of prostaglandin E2 restores defective lymphocyte proliferation and cell-mediated lympholysis in recipients after allogeneic marrow grafting. Blood 68:102–107
Koo HJ, Lim KH, Jung HJ, Park EH (2006) Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J Ethnopharmacol 103:496–500
Lai PKK, Chan JYW, Wu SB et al (2014) Anti-inflammatory activities of an active fraction isolated from the root of Astragalus membranaceus in RAW 264.7 Macrophages. Phytother Res 28:395–404
Li S, Long C, Liu F, Lee S, Guo Q, Li R (2006) Herbs for medicinal baths among the traditional Yao communities of China. J Ethnopharmacol 108:59–67
Litchfield JT, Wilcoxon F (1949) A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 96:99–135
Marotta P, Sautebin L, Di RM (1992) Modulation of the induction of nitric oxide synthase by eicosanoids in the murine macrophage cell line J774. Br J Pharmacol 107:640–641
Mukherjee H, Ojha D, Bag P, Chandel HS, Chawla Sarkar M, Chatterjee TH, Chakraborti S, Chattopadhyay D (2013) Antiherpes activities of a triterpene acid of Achyranthes aspera: an Indian ethnomedicine. Microbiol Res 168:238–244
Nag Chaudhuri AK, Karmakar S, Roy D, Pal S, Pal M, Sen T (2005) Anti-inflammatory activity of Indian black tea (Sikkim variety). Pharmacol Res 51:169–175
Pullaiah T, Naidu KC (2003) Antidiabetic plants in India and herbal based antidiabetic research. Regency, New Delhi
Rahmatullah M, Noman A, Hossan MS, Harun-Or-Rashid M, Rahman T, Chowdhury MH, Jahan R (2009) A survey of medicinal plants in two areas of Dinajpur district, Bangladesh including plants which can be used as functional foods. Am Eurasian J Sustainable Agric 3: 862–876. ISSN 1995-0748
Schiatti P, Selva D, Galliani G, Baldoli E, Diena A, Leali M, Toja E (1986) Highly selective anti-inflammatory and analgesic activity of 3-(1-methylethyl)-2-(4-methoxyphenyl)-3H-naphth [1, 2-d]imidazole, a new non-acidic molecule. Arzneim Forsch 36:102–109
Srinivas Reddy K, Rajeev Reddy E, Ganapaty S (2011) Diuretic and anti-inflammatory activity of aqueous extract of Aerva sanguinolenta (L.) Blume. Int Res J Pharm 2: 65–67. http://www.irjponline.com
Sur TK, Pandit S, Bhattacharya DK, Ashok CK, Mohanlakshmi S, Chattopadhyay D, Mondal SC (2002) Studies on anti-inflammatory activity of Betula alnoides bark. Phytother Res 16(7):669–671
Trebino CE, Stock JL, Gibbons CP et al. (2003) Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci USA. 100(15): 9044–9049
Williams TJ, Morley J (1973) Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature 246:215–217
Williams CS, Mann M, DuBois RN (1999) The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 18:7908–7916
Winter CA, Porter CA (1957) Effect of alterations in side chain upon anti-inflammatory and liver glycogen activities of hydrocortisone esters. J Am Pharm Assoc 46:515–519
Yamauchi R, Aizawa K, Inakuma T, Kato K (2001) Analysis of molecular species of glycolipids in fruit pastes of red bell pepper (Capsicum annuum L.) by high performance liquid chromatography-mass spectrometry. J Agric Food Chem 49:622–627
Acknowledgments
The authors are thankful to Dr. Basudeb Achari, former Emeritus Scientist, Indian Institute of Chemical Biology, India, for extending cooperation in structure elucidation of the isolated compound.
Conflict of interest
The authors declare that they have no conflict of interest.
Fundings
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mandal, A., Ojha, D., Lalee, A. et al. Bioassay directed isolation of a novel anti-inflammatory cerebroside from the leaves of Aerva sanguinolenta . Med Chem Res 24, 1952–1963 (2015). https://doi.org/10.1007/s00044-014-1261-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1261-0