Abstract
A series of novel 4-aminoquinoline derivatives were synthesized as antitumor agents by reacting 4-chloroquinoline with the corresponding mono/dialkyl amines. The cytotoxicity of these compounds was evaluated in vitro against HCT-116, A549, DU-145, HepG2, and LN229 cell lines. The results showed that most of the synthesized compounds displayed excellent cytotoxicity, and 5,7-dimethoxy-2-phenyl-N-propylquinoline-4-amine (6a) displayed the most potent cytotoxicity against HCT-116 cells. Furthermore, 6a could decrease VEGF protein expression.
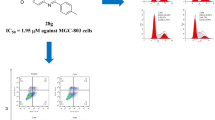
Graphical abstract
The synthesis and antitumor activity of 4-aminoquinoline derivatives have been described.



Similar content being viewed by others
References
Atwell GJ, Bos CD, Baguley BC, Denny WA (1988) Potential antitumor agents. 56. “Minimal” DNA-intercalating ligands as antitumor drugs: phenylquinoline-8-carboxamides. J Med Chem 31:1048–1052
Atwell GJ, Baguley BC, Denny WA (1989) Potential antitumor agents. 57. 2-Phenylquinoline-8-carboxamides as “Minimal” DNA-intercalating antitumor agents with in vivo solid tumor activity. J Med Chem 32:396–401
Cantin LD, Magnuson S, Gunn D, Barucci N, Breuhaus M, Bullock WH, Burke J, Claus TH (2007) PDE-10A inhibitors as insulin secretagogues. Bioorg Med Chem Lett 17:2869–2873
Chen YL, Huang CJ, Huang ZY, Tseng CH, Chang FS, Yang SH, Lin SR, Tzeng CC (2006) Synthesis and antiproliferative evaluation of certain 4-anilino-8-methoxy-2-phenylquinoline and 4-anilino-8-hydroxy-2-phenylquinoline derivatives. Bioorg Med Chem 14:3098–3105
Hennequin LF, Thomas AP, Johnstone C, Stokes ESE, Ple PA, Lohmann JJM, Ogilvie DJ, Dukes M, Wedge SR, Curwen JO, Kendrew J, Brempt CL (1999) Design and structure–activity relationship of a new class of potent VEGF receptor tyrosine kinase inhibitors. J Med Chem 42:5369–5389
Kakadiya R, Dong H, Kumar A, Narsinh D, Zhang XG, Chou TC, Lee TC, Shah A, Su TL (2010) Potent DNA-directed alkylating agents: synthesis and biological activity of phenyl N-mustard-quinoline conjugates having a urea or hydrazinecarboxamide linker. Bioorg Med Chem 18:2285–2299
Luo FT, Ravi VK, Xue CH (2006) The novel reaction of ketones with o-oxazoline-substituted anilines. Tetrahedron 62:9365–9372
Nilsson J, Nielsen EO, Liljefors T, Nielsen M, Sterner O (2008) Azaflavones compared to flavones as ligands to the benzodiazepine binding site of brain GABAA receptors. Bioorg Med Chem Lett 18:5713–5716
Sirisoma N, Pervin A, Zhang V, Jiang SC, Willardsen JA, Anderson MB, Mather G, Pleiman CM, Kasibhatla S, Tseng B, Drewe J, Cai SX (2010) Structure–activity relationship of the quinazoline ring. Bioorg Med Chem Lett 20:2330–2334
Solomon VR, Puri SK, Srivastava K, Katti SB (2005) Design and synthesis of new antimalarial agents from 4-aminoquinoline. Bioorg Med Chem 13:2157–2165
Solomon VR, Haq W, Smilkstein M, Srivastava K, Puri SK, Katti SB (2010) 4-Aminoquinoline derived antimalarials: synthesis, antiplasmodial activity and heme polymerization inhibition studies. Eur J Med Chem 45:4990–4996
Strekowski L, Say M, Henary M, Ruiz P, Manzel L, Macfarlane DE, Bojarski AJ (2003) Synthesis and activity of substituted 2-phenylquinolin-4-amines, antagonists of immunostimulatory CpG-oligodeoxynucleotides. J Med Chem 46:1242–1249
Xia Y, Yang ZY, Xia P, Hackl T, Hamel E, Mauger A, Wu JH, Lee KH (2001) Antitumor agents. 211. Fluorinated 2-phenyl-4-quinolone derivatives as antimitotic antitumor agents. J Med Chem 44:3932–3936
Zhang HW, Solomon VR, Hu CK, Ulibarri G, Lee H (2008) Synthesis and in vitro cytotoxicity evaluation of 4-aminoquinoline derivatives. Biomed Pharmacother 62:65–69
Zhao YL, Chen YL, Chang FS, Tzeng CC (2005) Synthesis and cytotoxic evaluation of certain 4-anilino-2-phenylquinoline derivatives. Eur J Med Chem 40:792–797
Acknowledgments
We sincerely thank the financial supports from the National Natural Science Foundation of China (Grant No. 21202012), 2010 Industry for Attracting Ph.D. Scientists Program of Jiangsu Province and Changzhou Key Technology R&D Program (Society Development).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ren, J., Zhao, J., Zhou, YS. et al. Synthesis and antitumor activity of novel 4-aminoquinoline derivatives. Med Chem Res 22, 2855–2861 (2013). https://doi.org/10.1007/s00044-012-0283-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0283-8