Abstract
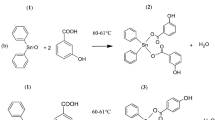
A new series of eleven novel 1-(3-chloro-2-oxo-4-phenylazetidin-1yl)-3-(2-oxo-2-(10H-phenothiazin-10-yl)ethyl)urea derivatives were synthesized by cyclocondensation of various Schiff bases of phenothiazine with chloroacetyl chloride in the presence of triethylamine. Various Schiff bases of phenothiazine were synthesized by condensation of 4-(2-oxo-2-(10H-phenothiazin-10-yl)ethyl semicarbazide with various aryl aldehydes. The synthesized compounds were characterized by IR, MASS and 1H NMR spectral data and evaluated for in vitro antimicrobial, antitubercular, antioxidant and anticancer activities by disc diffusion method, MIC method, REMA, DPPH, FRAP and MTT assay method, respectively. All synthesized compounds showed moderate-to-significant anti-bacterial and anti-fungal activity and compound 4d, 4g, 4h and 4k showed good antioxidant activity with EC50 value of 55, 57, 56 and 47 μg/ml tested by DPPH method. The compounds 4j at a concentration of 10 μg/ml showed inhibition against the growth of Mycobacterium tuberculosis and 4f showed significant activity against human cervical cancer cell line with IC50 values of 18.26 μM.













Similar content being viewed by others
References
Aaron BB, Jay HK, Eric MF, Erica LA, Cristofer MP, Heather MW, Michael JR, Miguel, Sang SC, Yuehong W (2007) Synthesis and anti-tubercular activity of quaternized promazine and promethazine derivatives. Bioorg Med Chem Lett 17:1346–1348
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power of FRAP assay. Anal Biochem 239:70–76
Chopde HN, Pagadala R, Jetti V (2011) An efficient synthesis of novel bioactive azetidinones and thiazolidinones of 1, 5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one. Int J Pharm Biosci 2(1):19–22
Gautam N, Goyal K, Saini O, Kumar A, Gautam DC (2011) Synthesis and biological activity of substituted 3-fluoro/3-trifluoromethy10H-phenothiazines, its ribofuranosides and sulfones. J F Chem 132:420–426
Gildasio AS, Luciana MMC, Fernanda CFB, Ana LPM, Eliezer JB, Carlos AMF (2004) New class of potent anti-nociceptive and antiplatelet 10H-phenothiazine-1-acylhydrazone derivatives. Bioorg Med Chem 12:3149–3158
Ginnis MC, Rinaldi MG (2001) Antifungal drugs; mechanism of action, drug resistance, susceptibility testing and assays of activity in biological fluids. In: Lorian V (ed) Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, pp 223–281
Ihara M, Lu YT, Arai C, Ge JG et al (2011) Synthesis and in vitro antiprotozoal activities of water-soluble, inexpensive phenothiazinium chlorides. Dyes Pigments 89:44–48
Jubie S, Gowramma B, Muthal NK, Kalirajan R, Gomath S, Elango K (2009) Synthesis and antimicrobial evaluation of some 2-azetidinone derivatives. Int J ChemTech Res 1(2):153–157
Krsti M, Sovilj S, Sipka SG, Radosavljevi I, Evans, Borozan S, Santibanez J, Koci J (2011) New ruthenium(II) complexes with N-alkylphenothiazines: synthesis, structure, in vivo activity as free radical scavengers and in vitro cytotoxicity. Eur J Med Chem 45:3669–3676
Kumar VD, Gupta AK, Yadav YC (2010) Synthesis, characterization and antimicrobial activity of benzimidazolyl-phenothiazine derivatives. Int J Pharm Res 2(2):45–50
Kure Barbara, Morris MichealD (1976) Spectra of Phenothiazines and some pharmaceutical derivatives. Talanta 23:398–400
Martha K, Nectarios K, Leann T, Leslie WD (2002) Novel phenothiazine antimalarials: synthesis, antimalarial activity, and inhibition of the formation of β-haematin. Biochem Pharmacol 63:833–842
Martin A, Camacho M, Portaels F, Palomino JC (2003) Resazurin microtiter assay plate testing of Mycobacterium tuberculosis susceptibilities to second-line drugs: rapid, simple, and inexpensive method. Antimicrob Agents Chemother 47(11):3616–3619
Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS, Leitao SG (2000) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15:127–130
Mitchell MO, Bate AB, Kalin JH, Fooksman EM et al (2007) Synthesis and antitubercular activity of quaternized promazine and promethazine derivatives. Bioorg Med Chem 17:1346–1348
Monks A et al (1991) Feasibility of high flux anticancer drug screen using a diverse panel of cultured human tumour cell lines. J Natl Cancer Inst 83:757–766
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Plutal K, Jelen M, Morak-Modawska B, Artym J, Kocieba M, Zimecki M (2010) Anticancer activity of newly synthesized azaphenothiazines from NCI’s anticancer screening centre. Pharmacol Rep 62:319–332
Radadiya VR, Purohit DM, Patolia VN (2005) Synthesis and antimicrobial activity of 10 N-{[(aryl)-amino]-methyl}-3-methoxy-10, 10a-dihydro-4a-H-phenoyhiazine-9-carboxylic acid. Indian J Chem 44B:112–1114
Rajasekaran A, Periasamy M, Venkatesan (2010) Synthesis, characterization and biological activity of some novel azetidinones. J Dev Biol Tissue Eng 2(1):5–13
Rokade Y, Dongare N (2010) Synthesis and antimicrobial activity of some azetidinones derivative with the β-napthol. Rasayan J Chem 3(4):641–645
Sahoo U, Seth AK, Sen A, Dhanya B, Patel J, Chawla R (2010) Synthesis and characterization of certain novel azetidinone derivatives as antibacterial and antifungal agents. Res J Pharm Biol Chem Sci 1(2):102
Singh HP, Pandeya SN, Sharma CS (2011) Synthesis and pharmacological screening of some novel chalconyl derivatives of substituted phenyl semicarbazide. Med Chem Res 20(2011):74–80
Srivastava SK, Dua R, Srivastava SD (2010) Synthesis and antimicrobial activity of [N1-(N-substitutedarylidene-hydrazino)-acetyl]-2-methyl-imidazoles and [N1-(4-substituted aryl-3-chloro-2-oxo-1-azetidinyl-amino)-acetyl]-2-methyl-imidazoles. Proc Natl Acad Sci India Sect A Phy Sci 80:117–121
Upadhyay RK, Upadhyay MS, Jain S (2009) Synthesis and antimicrobial activity of 1-[2-(10-p-Chlorobenzyl)phenothiazinyl]-3-(substitutedaryl)-2-propen-1-ones. Eur J Med Chem 6:S254–S258
Wainwright M, Meegan K, Loughran C (2011) Phenothiazinium photosensitisers IX. Tetra- and pentacyclic derivatives as photoantimicrobial agents. Dyes Pigments 91:1–5
Walsh TJ, Melcher GP, Rinaldi MG, Lecciones J et al (1990) Trichosporon beigelii, an emerging pathogen resistant to ampothericin B. J Clin Microbiol 28:1616–1622
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajasekaran, A., Devi, K.S. Synthesis and biological evaluation of 1-(3-chloro-2-oxo-4-phenylazetidin-1-yl)-3-(2-oxo-2-(10H-phenothiazin-10-yl)ethyl)urea derivatives. Med Chem Res 22, 2578–2588 (2013). https://doi.org/10.1007/s00044-012-0255-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0255-z