Abstract
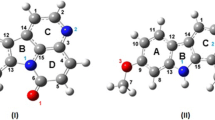
Structure optimization of two taxane diterpenoids I and II has been carried out using GAUSSIAN03 program with B3LYP/6-31G** basis set. The structures were fully optimized without any geometric constraints. Coordinates found in crystallographic study were used as input to the GAUSSIAN. The results show that the conformations of taxoid cores are almost same as were observed from crystallography, while the conformations of the substituents are slightly different. Thus, taxoid cores are robust enough to maintain their conformations in gaseous state while the conformations of substituents may vary depending upon the reaction condition. To understand the mode of binding, the docking studies of both compounds have been carried out with Russell’s viper phospholipase A2 (vPLA2) as target using induced fit docking. The docking results show that regarding the interactions and energy for indomethacin binding with vPLA2, compound I shows the best results.



Similar content being viewed by others
References
Appendino G (1993) Antimicrobial activity of the heartwood of Taxus baccata. Fitoterapia 64(suppl l):5–25
Appendino G, Gariboldi P, Gabetta B, Pace R, Bombardelli E, Viterbo D (1992) 14β-hydroxy-10-deacetyl baccatin III, a new taxane from Himalayan yew (Taxus wallichiana Zucc). J Chem Soc Perkin Trans I:2925–2930
Broughton HB (2000) A Method for including protein flexibility in protein-ligand docking: Improving tools for database mining and virtual screening. J Mol Graph Model 18:247–257
Chattopadhyay SK, Sharma RP, Appendino G, Gariboldi P (1995) A rearranged taxane from the Himalayan yew. Phytochemistry 39:869–870
Chattopadhyay SK, Sharon A, Yadava U, Srivastava S, Mehta VK, Maulik PR (2001) A taxane diterpenoid from the needles of Taxus wallichiana. Acta Cryst E57:o1158–o1160
Chattopadhyay SK, Sharon A, Yadava U, Srivastava S, Mehta VK, Maulik PR (2002) A taxane diterpenoid from the heartwood of Taxus wallichiana. Acta Cryst E58:o154–o155
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven JT, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) GAUSSIAN 03, Revision B.04, Gaussian, Inc., Pittsburgh
Glide (2010) Version 5.6. Schrodinger, LLC, New York, NY
Hartzell H Jr (1991) The yew tree: a thousand whispers. Hulogosi, Eugene, p 64
Jorgenson WL, Maxwell DS, Tirado-Rives J (1996) Development and testing of the OPLS all atom force field on conformational energetic and properties of organic liquids. J Am Chem Soc 118:11225–11236
Krussmann G (1983) Handbuch der Nadelgeholze. Paul Parey, Berlin and Hamburg, p 332
Mastropaolo D, Camerman A, Luo Y, Brayer GD, Camerman A (1995) Crystal and molecular structure of paclitaxel (taxol). Proc Natl Acad Sci USA 92:6920–6924
Moitessier N, Therrien E, Henessian S (2006) A method for induced fit docking scoring and ranking of flexible ligands. Application to peptidic and pseudopeptidic β-secretase (BACE 1) inhibitors. J Med Chem 49:5885–5894
Parmar VS, Vardhan A, Taneja P, Sinha R, Patnaik GK, Tripathi SC, Boll PM, Larsen S (1991) Absolute configuration of epirhododendrin and (−)–rhododendrin [=(−)–betuligenol] and X-ray crystal and molecular structure of rhododendron [=betuloside] a hepatoprotective constituent of Taxus baccata. J Chem Soc Perkin Trans 1:2687–2690
Pouvelle B, Farley PJ, Long CA, Taraschi TF (1994) Taxol arrests the development of blood stage Plasmodium falciparum, invitro and Plasmodium chabaudi adami in malaria infected mice. J Clin Invest 94:413–417
Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277:665–669
Sherman W, Day T, Jacobson MP, Friesner RA, Farid R (2006) Novel procedure for modelling ligand/receptor induced fit effects. J Med Chem 49:534–553
Singh N, Kumar RP, Kumar S, Sharma S, Mir R, Kaur P, Srinivasan A, Singh TP (2009) Simultaneous inhibition of anti-coagulation and inflammation: crystal structure of phospholipase A2 complexed with indomethacin at 1.4 Å resolution reveals the presence of the new common ligand-binding site. J Mol Recog 22(6):437–445
Stierle A, Strobel G, Stierle D (1993) Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of pacific yew. Science 260:214–216
Taylor RD, Jewsbury PJ, Essex JW (2002) A review of protein small molecule docking methods. J Comp Aided Mol Des 16:151–166
Vohora SB, Kumar I (1971) Studies on Taxus baccata—I, preliminary phytochemical and behavioral investigations. Planta Med 20:100–107
Vohora SB, Kumar I, Shah SA, Khan MSY (1980) Effect of biflavonoids of Taxus baccata on the central nervous system. Indian J Med Res 71:815–820
Vosco SH, Wilk L, Nusair M (1980) Accurate spin dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can J Phys 58:1200–1211
Zhang LU, Gallicchio E, Friesner RA, Levy RM (2001) Solvent models for protein ligand binding: comparison of implicit solvent poisson and surface generalized born models with explicit solvent simulations. J Comp Chem 22:591–607
Acknowledgments
U. Yadava is thankful to DST, New Delhi for financial assistance through SERC Fast Track Scheme for Young Scientists (Ref No. SR/FT/CS-78/2010). Authors are also grateful toward Prof. D. Velmurugan, Centre for Advanced Studies in Crystallography and Biophysics, Madras University, Chennai for his valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yadava, U., Gupta, H. & Roychoudhury, M. A comparison of crystallographic and DFT optimized geometries on two taxane diterpenoids and docking studies with phospholipase A2. Med Chem Res 21, 2162–2168 (2012). https://doi.org/10.1007/s00044-011-9724-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9724-z