Abstract
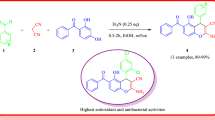
A series of substituted imidazoles have been synthesized under solvent-free condition by grinding 1,2-diketone, aromatic aldehyde, and ammonium acetate in the presence of molecular iodine as the catalyst. The short reaction time and easy workup make this protocol practically and economically attractive and are characterized by NMR spectra, X-ray, mass, and CHN analysis. Their antioxidant potential were evaluated using different in vitro antioxidant models namely, DPPH (1,1-diphenyl-2-picrylhydrazyl) radical, superoxide anion, and hydroxyl radical scavenging activities. Their antibacterial screening against Staphylococcus aureus, Escherichia coli, and Klbesiella pneumoniae and antifungal activity against Aspergillus niger, Aspergillus flavus, and Candida-6 were also evaluated. Among all, dimethoxyphenyl substituent at N3 of the imidazole derivatives exhibited the highest hydroxy and superoxide anion radical scavenging activities, whereas dimethoxyphenyl substituent at N3 and fluorophenyl at C2 of the imidazole derivatives exhibited the highest DPPH radical scavenging activity.










Similar content being viewed by others
References
Aruoma OI (1998) Free radicals, oxidative stress, and antioxidants in human health and diseases. J Am Oil Chem Soc 75:199–212
Baricevic D, Sosa S, Della Loggia R, Tubaro A, Simonovska B, Krasna A, Zupancic A (2001) Topical anti-inflammatory activity of Salvia officinalis L. leaves: the relevance of ursolic acid. J Ethnopharmacol 75:125–132
Branen AL (1975) Toxicology and biochemistry of butylated hydroxyanisol and butylated hydroxytoluene. J Am Oil Chem Soc 5:59–63
Clavin M, Gorzalczany S, Macho A, Munoz E, Ferraro G, Acevedo C, Martino V (2007) Anti-inflammatory activity of flavonoids from Eupatorium arnottianum. J Ethnopharmacol 112:585–589
Cook NC, Samman S (1996) Flavonoids-chemistry, metabolism, cardio-protective effects, and dietary sources. J Nutr Biochem 7:66–76
Duh PD (1998) Antioxidant activity of budrock (Arctium lappa Linn): it’s scavenging effect on free radical and active oxygen. J Am Oil Chem Soc 75:455–461
Elizabeth K, Rao MNA (1990) Oxygen radical scavenging activity of curcumin. Int J Pharm 58:237–240
Frautchy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, Cole GM (2001) Phenolic anti-inflammatory antioxidant reversal of a beta-induced cognitive deficits and neuropathology. Neurobiol Aging 22:993–1005
Gayathri P, Jayabharathi J, Saravanan K, Thiruvalluvar A, Butcher RJ (2010) 2-(4-Fluorophenyl)-1-(4-methoxyphenyl)-4, 5-dimethyl-1H-imidazole. Acta Cryst E66:o1791
Gordon MH (1990) The mechanism of the antioxidant action in vitro. In: Hudson BJF (ed) Food antioxidants. Elsevier, London, pp 1–18
Grice HP (1988) Enhanced tumour development by butylated hydroxyanisole (BHA) from the prospective of effect on fore-stomach and oesophageal squamous epithelium. Food Chem Toxicol 26:717–723
Gutteridgde JMC (1995) Free radicals in disease processes: a complication of cause and consequence. Free Rad Res Comm 19:141–158
Halliwell B (2000) The antioxidant paradox. Lancet 355:1179–1180
Jayabharathi J, Manimekalai A, Consalata Vani T, Padmavathy M (2007) Synthesis, stereochemistry and antimicrobial evaluation of t(3)-benzyl-r(2), c(6)-diarylpiperidin-4-one and its derivatives. Eur J Med Chem 42:60–593
Jayabharathi J, Thanikachalam V, Thangamani A, Padmavathy M (2008) Synthesis, AM1 calculation, and biological studies of thiopyran-4-one and their azine derivatives. Med Chem Res 16:266–279
Jayabharathi J, Thanikachalam V, Saravanan K (2009) Effect of substituents on the photoluminescence performance of Ir(III) complexes: synthesis, electrochemistry and photophysical properties. J Photochem Photobiol A Chem 208:13–20
Jayabharathi J, Thanikachalam V, Saravanan K, Srinivasan N (2010) Iridium(III) Complexes with orthometalated phenylimidazole ligands subtle turning of emission to the saturated green colour. J Fluoresc. doi:10.1007/s10895-010-0737-7
Jayabharathi J, Manimekalai A, Padmavathy M (2010) Synthesis, spectral, theoretical and antimicrobial screening of some heterocyclic oximes. Med Chem Res. doI: 10.1007/s00044-010-9427-x
Jianwei S, Dong Y, Cao L, Wang X, Wang S, Hu YY (2004) Highly efficient chemoselective deprotection of O, O-acetals and O, O-ketals catalyzed by molecular iodine in acetone. J Org Chem 69:8932–8934
Koleva II, Van Beek TA, Linssen JPH, de Groot A, Evstatieva LN (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal 13:8–17
Lambardino JG, Wiseman EH (1974) Preparation and antiinflammatory activity of some nonacidic trisubstituted imidazoles. J Med Chem 17:1182–1188
Lantos I, Zhang W, Shiu X, Eggleston DS (1993) Synthesis of imidazoles via hetero-Cope rearrangements. J Org Chem 58:7092–7095
Maier T, Schmierer R, Bauer K, Bieringer H, Buerstell H, Sachse B (1989) US Patent 820335. Chem Abstr 111:19494
Mantle D, Eddeb F, Pickering AT (2000) Comparison of relative antioxidant activities of British medicinal plant species in vitro. J Ethnopharmacol 72:47–51
Nishimiki M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Comm 46:849–853
Oke JM, Hamburger MO (2002) Screening of some Nigerian medicinal plants for antioxidant activity using 2,2-diphenyl-picryl-hydrazyl radical. Afr J Biomed Res 5:77–79
Okhawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem 95:351–355
Oyaizu M (1986) Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 44:307–315
Parejo I, Viladomat F, Bastida J, Rosas-Romero A, Saavedra G, Murcia MA, Jimenez AM, Codina C (2003) Investigation of Bovilian plant extracts for their radical scavenging activity and antioxidant activity. Life Sci 73:1667–1681
Ren P, Liu T, Qin J, Chen C (2003) A new approach to suppress nonlinearity-transparency trade-off through coordination chemistry: syntheses and spectroscopic study on second-order nonlinear optical properties of a series of square-pyramidal zinc(II) complexes. Spectrochim Acta A 59:1095–1101
Robak J, Gryglewski RJ (1998) Flavonoids are scavengers of aqueous phase radicals and as superoxide anions. Biochem Pharmacol 37:837–841
Saravanan K, Srinivasan N, Thanikachalam V, Jayabharathi J (2010) Synthesis and photo physics of some novel imidazole derivatives used as sensitive fluorescent chemisensors. J Fluoresc. doi:10.1007/s10895-010-0690-5
Schuler P (1990) Natural antioxidants exploited commercially. In: Hudson BJF (ed) Food Antioxidants. Elsevier, London, pp 99–170
Valentao P, Fernndes E, Canvalho E, Andrade PB, Seabra RM, Bastos ML (2002) Studies on the antioxidant activity of Lippia citriodora infusion: scavenging effect on superoxide radical, hydroxyl radical and hypochlorous acid. Biol Pharm Bull 25:1324–1327
Wang L, Tu YC, Lian TW, Hung JT, Yen JH, Wu MJ (2006) Distinctive antioxidant and anti-inflammatory effects of flavonols. J Agric Food Chem 54:9798–9804
Wichi HC (1986) Safety evaluation of butylated hydroxytoluene (BHT) in the liver, lung and gastrointestinal tract. Food Chem Toxicol 24:1127–1130
Zhang C, Moran EJ, Woiwade TF, Short KM, Mjalli AM (1996) Synthesis of tetrasubstituted imidazoles via α-(N-acyl-N-alkylamino)-β-ketoamides on Wang resin. Tetrahedron Lett 37:751–754
Acknowledgments
One of the authors, Dr. J. Jayabharathi, Associate Professor in Chemistry, Annamalai University is thankful to the Department of Science and Technology (No. SR/S1/IC-07/2007), the University Grants Commission (F. No. 36-21/2008 (SR)) for providing fund to this research study, and Prof. C.H. Cheng, Chairman, the National Science Council, the National Tsing Hua University for her post-doctoral research study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayabharathi, J., Thanikachalam, V., Rajendraprasath, N. et al. Antioxidant potential and antimicrobial screening of some novel imidazole derivatives: greenway efficient one pot synthesis. Med Chem Res 21, 1850–1860 (2012). https://doi.org/10.1007/s00044-011-9702-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9702-5