Abstract
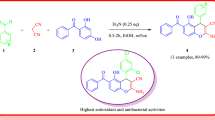
A series of new coumarin derivatives 4 containing a chalcone moiety were synthesized by condensation of 3-acetyl-4-hydroxycoumarin 1 with aryl or heteroaryl aldehydes 2 in the presence of piperidine in chloroform. The interaction of 3-formyl-4-chloro-coumarin 3 with nitrogen compound nucleophiles are described and lead to the corresponding substituted chromen[4,3-c] pyrazol-4-ones 5. The structures of the obtained compounds were established on the basis of IR|1D|2D NMR, while crystal structure of 3-acetyl-4-hydroxy coumarin 1 was determined using X-ray diffraction and further were evaluated for possible antibacterial and antioxidant activities. The coumarinic chalcone 4d has been found to be the most active (IC50 = 2.07 μM) in this study.









Similar content being viewed by others
Abbreviations
- LTR:
-
Long terminal repeat
- TPA:
-
Two photon absorption
- AIDS:
-
Acquired immunodeficiency syndrome
References
Antonio R, Hedi Z, Chaker L, Hamdi N, Mustapha S (2006) Synthesis of some new biologically active coumarin derivates. Chem Heterocycl Comp 3:361–366
Antonio R, Hamdi N, Mustapha S (2008) Synthesis, spectroscopic and antibacterial investigations of new hydroxyl ethers and heterocyclic coumarin derivates. J Heterocycl Chem 45:1835–1842
Artamonova OS, Chernyakova IV, Malysheva LI, Pryanishnikova NT, Savel’ev VL, Shavyrina VV, Zagorevskii VA (1983) Synthesis and pharmacological activity of 4H-(1)-benzopyrano-(3,4-d)-imidazol-4-ones. Khim Farm Zh 17:697–700 (see Chem Abstr 99:158–325)
Bordin F, Chilin A, Dall’Acqua F, Guiotto A, Manzini P (1995) Synthesis of angelicin heteroanalogues: preliminary photobiological and pharmacological studies. Rod Il Farm 50:479–488
Borrow E, Barrow W, Flavin MT, Lin YM, Suling WJ, Xu ZQ, Westbrook L (2004) Chemical resolution of (±)-calanolide A, (±)-cordatolide A and their 11-demethyl analogues. Bioorg Med Chem 12:1199–1207
Brown SA, Mendez J, Murray DH (1982) The natural coumarins. Occurrence, chemistry and biochemistry. Wiley, New York
Carmen P, Hamdi N, Pedro V (2008) Synthesis, structure, antimicrobial and antioxidant investigations of dicoumarol and related compounds. Eur J Med Chem 43:2541–2548
Dall’Acqua F, Horspool WM, Vedaldi D, Song PS (1995) Handbook of organic photochemistry and photobiology. CRC Press, Boca Raton, pp 1341–1350
David AL, Michael TH (2001) A C−H···O=C hydrogen bond intramolecular hydrogen bonding in a novel semirubin. J Org Chem 66:8402–8410
Dholakia VN, Parekh MG, Trivedi NK (1968) Improved and rapid synthesis of new coumarinyl chalcone derivatives and their antiviral activity. Aust J Chem 22:345–2347
Eggenweiler M, Gassen M, Rochus J, Poeschke O, Wolf M (2001) Merck Patent Gmbh, Germany, PCT Int. Appl. (see: Chem Abstr 134:331–619)
Evans JM, Geen GR, Vong AK (1996) In: McKillop A (ed) Pyrans and their benzo derivatives: applications. comprehensive heterocyclic chemistry II. Pergamon, Oxford, pp 469–484
Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN (2004) Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr Pharm 10:3813–3833
Hamdi N, Sakellariou R, Speziale V (1992) Antibacterial, antifungal and cytotoxic properties of novel N-substituted sulfonamides from 4-hydroxycoumarin. J Heterocycl Chem 29:1817
Hamdi M, Reine S, Patrick G, Vincent (1993) Reaction of amines on 3-ureidomethylenecoumarins. A new route to N-(methylene-4-oxocoumarinyl) amines. J Hetercycl Chem 30:1155–1157
Hantschmann A, Liebigs M, Steinfuhrer T, Weissenfels M (1992) Ann Chem 23
Hatano T, Kagawa H, Okuda T, Yasuhara T (1988) Antioxidant activity and volatile components of Egyptian Artemisia judaica L. Chem Phar Bull 36:2090–2097
Hatano T, Ito H, Tanuma S, Uchiumi F, Yoshida T (2003) Transcriptional suppression of the HIV promoter by natural compounds. Antiviral Res 58:89–98
Heber D, Ivanov IC, Karagiasov SK (1995) J Heterocycl Chem 32:505
Ivanov IC, Karagiosov SK, Simeonov MF (1992) A facile synthesis of benzopyrano[4,3-b]pyridin-5-ones. Liebigs Ann Chem 203–207
Joshi HS, Kachhadia VV, Nimavat KS, Potal KH (2003) Improved and rapid synthesis of new coumarinyl chalcone derivatives and their antiviral activity. J Ind Chem Soc 80:707–708
Kadaba PK, Tisler M, Stanovnik B (1984) 1,2,3-Triazole and its derivatives: development of methods for the formation of the triazole ring. Adv Heterocycl Chem 37:339
Kalaj V, Kitan D, Trkovnik M (1987) Synthesis of new heterocyclocoumarins from 3,4-damino-and 4-chloro-3-nitrocoumarins. Org Prep Proc Int 19:450–455
Li X, Zhao Y, Wang T, Shi M, Wu F (2007) Coumarin derivatives with enhanced two-photon absorption cross-sections. Dyes Pigments 74:108–112
Mahidol C, Ploypradith P, Sahakitpichan P, Wongbundit S, Ruchirawat (2004) A Highly efficient synthesis of Lamellarins K and L by the Michael addition/ring-closure reaction of benzyldihydroisoquinoline derivatives with ethoxycarbonyl-β-nitrostyrenes. Angew Chem Int 43:866–868
Malhotra S, Parmar VS, Sharma VK (1988) Synthesis of three new dihydropyranochalcones: structural revision of crotmadine, an antifungal constituent of Crotalaria madurensis. J Nat Prod 51:578–581
NCCLS (1997) Methods for dilution antimicrobial susceptibility. Test for bacteria that grow aerobically, 4th ed, Approved Standard. NCCLS Document M7-A4. NCCLS, Villanowa, PA
O’Kennedy R, Thornes RD (1997) Coumarins: biology, applications and mode of action. Wiley, Chichester
Oh C, Park KP (1994) J Heterocycl Chem 31:841
Palinko I, Toroka BB, Rozsa-Tarjanyi M, Kiss JT, Tasi GY (1995) Hydrogen bonding interactions of α-phenylcinnamic acid isomers in the liquid phase studied by IR and NMR spectroscopies and computational methods. J Mol Struct 348:57–60
Tabakovic′ K, Tabakovic′ I (1981) Chemistry of coumarins. Nucleophilic substitutions of chloro-3-nitrocoumarin with hard and soft nucleophiles. Croat Chem Acta 54:451–458
Acknowledgements
This work was carried out with financial aid of both Tunisian Ministry of Higher Education and Scientific Research and Technology and the Spanish Agency of International Cooperation through projects (A/9549/07and A/8302/07).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamdi, N., Fischmeister, C., Puerta, M.C. et al. A rapid access to new coumarinyl chalcone and substituted chromeno[4,3-c]pyrazol-4(1H)-ones and their antibacterial and DPPH radical scavenging activities. Med Chem Res 20, 522–530 (2011). https://doi.org/10.1007/s00044-010-9326-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-010-9326-1