Abstract
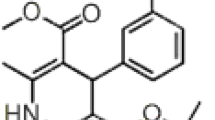
Fexofenadine is a non-sedative and selective peripheral H1 receptor antagonist prescribed for allergic rhinitis and chronic urticaria. This article deals with a simple, feasible, and sensitive isocratic reverse-phase high-performance liquid chromatographic method for the determination of fexofenadine hydrochloride in bulk drug, pharmaceutical dosage forms and in human serum. The chromatography was carried out at 20 ± 2°C using two different chromatographs and five different stationary phases. The isocratic mobile phase was phosphate buffer pH 7.4 and methanol (methanol–phosphate buffer, 35:65, v/v), detection was made at 218 nm and the mobile phase flowed at 1 ml min−1. Validation parameters included linearity, accuracy, precision, specificity, limit of detection (LOD), limit of quantification (LOQ), and robustness over a linearity range 5–15 μg ml−1 according to the ICH guidelines (r > 0.9999), the inter- and intra-day precisions were relative standard deviation (RSD) < 0.8%. The system suitability was scrutinized by capacity factor, tailing factor, and number of theoretical plates (capacity factor > 2.0, tailing factor ≤ 2.0, and theoretical plates > 2000). The retention time for five different stationary phases ranged from 3.78 to 4.15 min. The LOD and LOQ for the procedure were executed on samples containing very low concentrations of analytes on two different commercial brands of detectors.










Similar content being viewed by others
References
Arayne MS, Sultana N, Siddiqui FA (2005) Determination and quantification of cetirizine HCl in dosage formulations by RP-HPLC. Pak J Pharm Sci 18:7–11
Arayne MS, Sultana N, Nawaz M (2008) Simultaneous quantification of cefpirome and cetirizine or levocetirizine in pharmaceutical formulations and human plasma by RP-HPLC. J Anal Chem 63:881–887
Barnes CL, McKenzie CA, Webster KD, Poinsett-Holmes K (1993) Cetirizine: a new, non-sedating antihistamine. Ann Pharmacother 27:464–470
British Pharmacoepia (2000) Her Majesty Stationary Office, pp 342–343
Caballero E, Ocaña I, Azanza JR, Sádaba B (1999) Fexofenadine: a antihistaminic review of its practical characteristics. Rev Med Univ Navarra 43:93–97
CDER: Center for Drug Evaluation and Research (1994) Reviewer guidance for validation of chromatographic methods. Disponívelem: http://www.fda.gov/cder/guidance/cmc3.pdf
Drescher S, Schaeffeler E, Hitzl M, Hofmann U, Schwab M, Brinkmann U, Eichelbaum M, Fromm MF (2002) MDR1 gene polymorphisms and disposition of the P-glycoprotein substrate fexofenadine. Br J Clin Pharmacol 53:526–534
Fu I, Woolf EJ, Matuszewski BK (2004) Determination of fexofenadine in human plasma using 96-well solid phase extraction and HPLC with tandem mass spectrometric detection. J Pharm Biomed Anal 35:837–846
Gazy AA, Mahgoub H, El-Yazbi FA, El-Sayed MA, Youssef RM (2002) Determination of some histamine H1 receptor antagonists in dosage forms. J Pharm Biomed Anal 30:859–867
Gergov M, Robson JN, Ojanperä I, Heinonen OP, Vuori E (2001) Simultaneous screening and quantification of 18 antihistamine drugs in blood by liquid chromatography ionspray tandem mass spectrometry. Forensic Sci Int 121:108–115
Gowekar NM, Pande VV, Kasture AV, Tekade AR, Chandorkar JG (2007) Spectrophotometric estimation of ambroxol and cetirizine hydrochloride from tablet dosage form. Pak J Pharm Sci 20:250–251
Hamman MA, Bruce MA, Haehner-Daniels BD, Hall SD (2001) The effect of rifampin administration on the disposition of fexofenadine. Clin Pharmacol Ther 69:114–121
ICH guidelines Topic Q2 (R1) Validation of analytical procedures: methodology. www.emea.europa.eu/htms/human/ich/ichquality.htm.
Inomata N, Tatewaki S, Ikezawa Z (2009) Multiple H1-antihistamine-induced urticaria. J Dermatol 36:224–227
Karakuş S, Küçükgüzel I, Küçükgüzel SG (2008) Development and validation of a rapid RP-HPLC method for the determination of cetirizine or fexofenadine with pseudoephedrine in binary pharmaceutical dosage forms. J Pharm Biomed Anal 46:295–302 [Epub 2007 October 22]
Markham A, Wagstaff AJ (1998) Fexofenadine. Drugs 55:269–274 discussion 275–276
Mattila MJ, Paakkari I (1999) Variations among non-sedating antihistamines: are there real differences? Eur J Clin Pharmacol 55:85–93
Milne RW, Larsen LA, Ãrgensen KLJ, Bastlund J, Stretch GR, Evans AM (2000) Hepatic disposition of fexofenadine: influence of the transport inhibitors erythromycin and dibromosulphothalein. Pharm Res 17:1511–1515
Naidong W, Shou WZ, Addison T, Maleki S, Jiang X (2002) Liquid chromatography/tandem mass spectrometric bioanalysis using normal-phase columns with aqueous/organic mobile phases—a novel approach of eliminating evaporation and reconstitution steps in 96-well SPE. Rapid Commun Mass Spectrom 16:1965–1975
Pathak SM, Kumar AR, Musmade P, Udupa N (2008) A simple and rapid high performance liquid chromatographic method with fluorescence detection for the estimation of fexofenadine in rat plasma—application to preclinical pharmacokinetics. Talanta 76:338–346 [Epub 2008 March 13]
Pratt C, Brown AM, Rampe D, Mason J, Russell T, Reynolds R, Ahlbrandt R (1999) Cardiovascular safety of fexofenadine HCl. Clin Exp Allergy 29:212–216
Radhakrishna T, Om Reddy G (2002) Simultaneous determination of fexofenadine and its related compounds by HPLC. J Pharm Biomed Anal 29:681–690
Russell T, Stoltz M, Weir S (1998) Pharmacokinetics, pharmacodynamics, and tolerance of single- and multiple-dose fexofenadine hydrochloride in healthy male volunteers. Clin Pharmacol Ther 64:612–621
Simpson K, Jarvis B (2000) Fexofenadine: a review of its use in the management of seasonal allergic rhinitis and chronic idiopathic urticaria. Drugs 59:301–321
Sultana N, Arayne MS, Shamshad H (2009) In vitro studies of the interaction between cetirizine and H2 receptor antagonists using spectrophotometry and reversed-phase high-performance liquid chromatography. Med Chem Res. doi:10.1007/s00044-009-9204-x
Uno T, Yasui-Furukori N, Takahata T, Sugawara K, Tateishi T (2004) Liquid chromatographic determination of fexofenadine in human plasma with fluorescence detection. J Pharm Biomed Anal 35:937–942
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arayne, M.S., Sultana, N., Shehnaz, H. et al. RP-HPLC method for the quantitative determination of fexofenadine hydrochloride in coated tablets and human serum. Med Chem Res 20, 55–61 (2011). https://doi.org/10.1007/s00044-009-9285-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-009-9285-6