Abstract
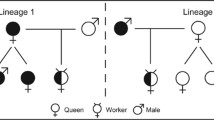
Asexual reproduction and hybridisation are often found among highly invasive plants and marine invertebrates. Recently, it has been suggested that clonality may enhance the success of invasive ants. In contrast, obligate hybridisation (dependent lineage genetic caste determination or DL GCD in ants) may decrease the chances of population persistence if one lineage is less prevalent than the other (asymmetry in lineage ratio). Genetic data available for the invasive yellow crazy ant (Anoplolepis gracilipes) suggest that it has an unconventional mode of reproduction that may involve asexual reproduction by workers or queens, or a form of genetic caste determination. Here, we investigated whether A. gracilipes reproduction involved DL GCD. The potential for worker reproduction was also assessed. We used microsatellite markers to assess the population structure of A. gracilipes workers, males, queens and sperm in queen spermathecae, from field collections in Arnhem Land. We found that a single queen lineage is present in Arnhem Land. The presence of a single lineage of queens discounts the possibility of DL GCD. Population structure separated queens and workers into different lineages, suggesting that these castes are determined genetically in A. gracilipes, or the mode of reproduction differs between workers and queens. Evidence for worker reproduction was weak. We conclude that the reproductive mode of A. gracilipes does not involve DL GCD. The resolution of the reproductive mode of A. gracilipes is complicated by a high prevalence of diploid males. The determination of the A. gracilipes reproductive mode remains a fascinating research question, and its resolution will improve our understanding of the contribution of the reproductive system to invasion success.




Similar content being viewed by others
References
Anderson K.E., Hölldobler B., Fewell J.H., Mott B.M. and Gadau J. 2006. Population-wide lineage frequencies predict genetic load in the seed-harvester ant Pogonomyrmex. Proc. Natl Acad. Sci. USA 103: 13433–13438
Arnaud-Haond S. and Belkhir K. 2007. Genclone: a computer program to analyse genotypic data, test for clonality and describe spatial clonal organization. Mol. Ecol. Notes 7: 15–17
Balloux F., Lehmann L. and de Meeûs T. 2003. The population genetics of clonal and partially clonal diploids. Genetics 164: 1635–1644
Bourke A.F.G. and Franks N.R. 1995. Social Evolution in Ants. Princeton University Press, Princeton, NJ
Cournault L. and Aron S. 2009. Diploid males, diploid sperm production, and triploid females in the ant Tapinoma erraticum. Naturwissenschaften 19: 1393–1400
Crozier R.H. 1971. Heterozygosity and sex determination in haplo-diploidy. Am. Nat. 105: 399–412
Dobata S., Shimoji H., Ohnishi H., Hasegawa E. and Tsuji K. 2012. Paternally inherited alleles in male body parts of an ant (Diacamma sp.) sex mosaic: implication for androgenetic male production in the Hymenoptera. Insect. Soc. 59: 55–59
Drescher J., Blüthgen N. and Feldhaar H. 2007. Population structure and intraspecific aggression in the invasive ant species Anoplolepis gracilipes in Malaysian Borneo. Mol. Ecol. 16: 1453–1465
Facon B., Genton B.J., Shykoff J., Jarne P., Estoup A. and David P. 2006. A general eco-evolutionary framework for understanding bioinvasions. Trends Ecol. Evol. 21: 130–135
Feldhaar H., Drescher J. and Blüthgen N. 2006. Characterization of microsatellite markers for the invasive ant species Anoplolepis gracilipes. Mol. Ecol. Notes 6: 912–914
Feldhaar H., Foitzik S. and Heinze J. 2008. Lifelong commitment to the wrong partner: hybridization in ants. Philos. Trans. R. Soc. B 363: 2891–2899
Foucaud J., Orivel J., Fournier D., Delabie J.H.C., Loiseau A., Le Breton J., Cerdan P. and Estoup A. 2009. Reproductive system, social organization, human disturbance and ecological dominance in native populations of the little fire ant, Wasmannia auropunctata. Mol. Ecol. 18: 5059–5073
Fournier D., Estoup A., Orivel J., Foucaud J., Jourdan H., Breton J.L. and Keller L. 2005. Clonal reproduction by males and females in the little fire ant. Nature 435: 1230–1234
Gao X. and Starmer J. 2008. AWclust: point-and-click software for non-parametric population structure analysis. BMC Bioinformatics 9: 77
Gruber M.A.M., Hoffmann B.D., Ritchie P.A. and Lester P.J. 2012. Recent behavioural and population genetic divergence of an invasive ant in a novel environment. Divers. Distrib. 18: 323–333
Hedrick P.W. and Parker J.D. 1997. Evolutionary genetics and genetic variation of haplodiploids and x-linked genes. Annu. Rev. Ecol. Syst. 28: 55–83
Heinze J. 2008. The demise of the standard ant. Myrmecol. News 11: 9–20
Helms Cahan S., Parker J.D., Rissing S.W., Johnson R.A., Polony T.S., Weiser M.D. and Smith D.R. 2002. Extreme genetic differences between queens and workers in hybridizing Pogonomyrmex harvester ants. Proc. R. Soc. B 269: 1871–1877
Helms Cahan S. and Vinson S.B. 2003. Reproductive division of labor between hybrid and nonhybrid offspring in a fire ant hybrid zone. Evolution 57: 1562–1570
Hölldobler B. and Wilson E.O. 1990. The Ants. The Belknap Press of Harvard University Press, Cambridge, MA
Holway D.A., Lach L., Suarez A.V., Tsutsui N.D. and Case T.J. 2002. The causes and consequences of ant invasions. Annu. Rev. Ecol. Syst. 33: 181–233
Jones S.R. and Phillips S.A.J. 1985. Gynandromorphism in the ant Pheidole dentata Mayr (Hymenoptera, Formicidae). Proc. Entomol. Soc. Wash. 87: 583–586
Keller L. 2007. Uncovering the biodiversity of genetic and reproductive systems: time for a more open approach. Am. Nat. 169: 1–8
Kellner K. and Heinze J. 2010. Mechanism of facultative parthenogenesis in the ant Platythyrea punctata. Evol. Ecol. 25:77–89
Kobayashi K., Hasegawa E. and Ohkawara K. 2008. Clonal reproduction by males of the ant Vollenhovia emeryi (Wheeler). Entomol. Sci. 11: 167–172
Krieger M.J.B. and Keller L. 2000. Mating frequency and genetic structure of the Argentine ant Linepithema humile. Mol. Ecol. 9: 119–126
Liebert A.E., Sumana A. and Starks P.T. 2005. Diploid males and their triploid offspring in the paper wasp Polistes dominulus. Biol. Lett. 1: 200–203
Mack R.N., Simberloff D., Lonsdale W.M., Evans H., Clout M. and Bazzaz F.A. 2000. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10: 689–710
Mergeay J., Verschuren D. and De Meester L. 2006. Invasion of an asexual American water flea clone throughout Africa and rapid displacement of a native sibling species. Proc. R. Soc. B 273: 2839–2844
Ohkawara K., Nakayama M., Satoh A., Trindl A. and Heinze J. 2006. Clonal reproduction and genetic caste differences in a queen-polymorphic ant, Vollenhovia emeryi. Biol. Lett. 2: 359–363
Peakall R. and Smouse P.E. 2006. GenAlEx 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 6: 288–295
Pearcy M., Aron S., Doums C. and Keller L. 2004. Conditional use of sex and parthenogenesis for worker and queen production in ants. Science 306:1780–1783
Pearcy M., Goodisman M.A.D. and Keller L. 2011. Sib mating without inbreeding in the longhorn crazy ant. Proc. R. Soc. B 278: 2677–2681
Pearcy M., Hardy O. and Aron S 2006. Thelytokous parthenogenesis and its consequences on inbreeding in an ant. Heredity 96: 377–382
Peeters C. 1987. The reproductive division of labour in the queenless ponerine ant Rhytidoponera sp. 12. Insect. Soc. 34: 75–86
Pimentel D. 2005 Environmental consequences and economic costs of alien species. In: Invasive Plants: Ecological and Agricultural Aspects (Inderjit, Ed). Birkhäuser Verlag, Basel, Switzerland, pp 269–276
Pritchard J.K., Stephens M. and Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155: 945–959
Raymond M. and Rousset F. 1995. GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J. Hered. 86: 248–249
Ren M.X., Zhang Q.G. and Zhang D.Y. 2005. Random amplified polymorphic DNA markers reveal low genetic variation and a single dominant genotype in Eichhornia crassipes populations throughout China. Weed Res. 45: 236–244
Rousset F. 2008. GENEPOP’007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol. Ecol. Resour. 8: 103–106
Schuelke M. 2000. An economic method for the fluorescent labeling of PCR fragments. Nature 18: 233–234
Schwander T., Cahan S.H. and Keller L. 2006. Genetic caste determination in Pogonomyrmex harvester ants imposes costs during colony founding. J. Evol. Biol. 19: 402–409
Schwander T., Lo N., Beekman M., Oldroyd B.P. and Keller L. 2010. Nature versus nurture in social insect caste differentiation. Trends Ecol. Evol. 25: 275–282
Sepp R., Szabo I., Uda H. and Sakamoto H. 1994. Rapid techniques for DNA extraction from routinely processed archival tissue for use in PCR. J. Clin. Pathol. 47: 318–323
Simon J-C., Delmotte F., Rispe C. and Crease T. 2003. Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals. Biol. J. Linn. Soc. 79: 151–163
Suomalainen E., Saura A. and Lokki J. 1987. Cytology and Evolution in Parthenogenesis. CRC Press Inc., Boca Raton, FL
Thomas M., Becker K., Abbott K. and Feldhaar H. 2010. Supercolony mosaics: two different invasions by the yellow crazy ant, Anoplolepis gracilipes, on Christmas Island, Indian Ocean. Biol. Invasions 12: 677–687
van Oosterhout C., Hutchinson W.F., Willis D.P.M. and Shipley P. 2004. MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 4: 535–538
van Wilgenburg E., Driessen G. and Beukeboom L.W. 2006. Single locus complementary sex determination in Hymenoptera: an “unintelligent” design? Front. Zool. 3: 1–15
Volny V.P. and Gordon D.M. 2002. Genetic basis for queen-worker dimorphism in a social insect. Proc. Natl Acad. Sci. USA 99: 6108–6111
Wetterer J.K. 2005. Worldwide distribution and potential spread of the long-legged ant, Anoplolepis gracilipes (Hymenoptera: Formicidae). Sociobiology 45: 77–97
Yoshizawa J., Mimori K., Yamauchi K. and Tsuchida K. 2009. Sex mosaics in a male dimorphic ant Cardiocondyla kagutsuchi. Naturwissenschaften 96: 49–55
Zayed A. and Packer L. 2005. Complementary sex determination substantially increases extinction proneness of haplodiploid populations. Proc. Natl Acad. Sci. USA 102: 10742–10746
Acknowledgments
Early drafts of this manuscript benefitted from comments by Theo Evans, Joe Zuccarello, Julien Grangier, Allan Burne, Claudie Doums, Christian Peeters, Alexandra Sébastien, Sébastien Rioux-Paquette, Kazuki Tsuji and several reviewers. We thank Daryl Lacey from Dhimurru Aboriginal Corporation for assistance with sample collection. This work was funded and supported by the Victoria University of Wellington Research Trust. M. Gruber was financially supported by a New Zealand Tertiary Education Commission Top Achiever Doctoral Scholarship, and a Victoria University of Wellington Hunter Scholarship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gruber, M.A.M., Hoffmann, B.D., Ritchie, P.A. et al. The conundrum of the yellow crazy ant (Anoplolepis gracilipes) reproductive mode: no evidence for dependent lineage genetic caste determination. Insect. Soc. 60, 135–145 (2013). https://doi.org/10.1007/s00040-012-0277-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-012-0277-z