Abstract
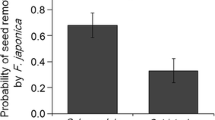
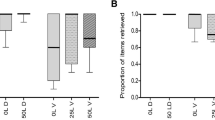
In contrast to other plant–animal mutualisms, seed dispersal interactions, and particularly seed dispersal by ants, are generally considered asymmetric, non-specialized relationships in which dispersers depend less on plants than vice versa. Although myrmecochory is well understood in many terrestrial ecosystems, dispersal of non-elaiosome-bearing seeds by ants has barely been studied outside the Neotropics. Aphaenogaster senilis, a common ant in Southern Spain, collects a great variety of non-myrmecochorous diaspores along with insect prey. At our study site, fleshy fruits of Arum italicum, Phillyrea angustifolia and Pistacia lentiscus represent up to one-fourth of the items collected by A. senilis from June to November. However, they are mostly ignored by other ants. In the laboratory, the addition of A. italicum fruits to A. senilis insect-based diet increased male production and both worker and queen pupae size. Seeds were transported up to 8 m away from the mother plant and deposited in a favorable habitat allowing a relatively high proportion of germination. Given important differences in seed production between species, our data suggest that A. senilis removes virtually all seeds of A. italicum, but a negligible fraction of P. lentiscus seeds. We conclude that in contrast to the common view, dispersal of non-myrmecochorous Mediterranean plants by ants might be an important phenomenon. Keystone disperser ants like A. senilis probably obtain an important fitness advantage from non-myrmecochorous diaspore collection. However, plant benefit may vary greatly according to the amount of seeds per individual plant and the existence of alternative dispersal agents.




Similar content being viewed by others
References
Alcántara J.M., Rey P.J., Manzaneda A.J., Boulay R., Ramírez J.M. and Fedriani J.M. 2007. Geographic variation in the adaptive landscape for seed size at dispersal in the myrmecochorous Helleborus foetidus. Evol. Ecol. 21: 411-430
Aranda-Rickert A. and Fracchia S. 2011. Pogonomyrmex cunicularius as the keystone disperser of elaiosome-bearing Jatropha excisa seeds in semi-arid Argentina. Entomol. Exp. Appl. 139: 91-102
Aronne G. and Wilcock C.C. 1994. First evidence of myrmecochory in fleshy fruited shrubs of the Mediterranean region. New Phytol. 127: 781-788
Bas J.M., Oliveras J. and Gomez C. 2009. Myrmecochory and short-term seed fate in Rhamnus alaternus: ant species and seed characteristics. Acta Oecol. 35: 380-384
Beattie A. and Hughes L. 2002. Ant-plant interactions. In: Plant Animal Interactions (Herrera C.M. and Pellmyr O., Eds). Blackwell Science, Oxford. pp 221-235
Böhning-Gaese K., Gaese B. and Rabemanantsoa S.B. 1999. Importance of primary and secondary seed dispersal in the malagasy tree Commiphora guillaumini. Ecology 80: 821-832
Bond W.J. and Slingsby P. 1984. Collapse of an ant-plant mutualism: the Argentine ant (Iridomyrmex humilis) and myrmecochorous Proteaceae. Ecology 65: 1031-1037
Bono J.M. and Heithaus E.R. 2002. Sex ratios and the distribution of elaiosomes in colonies of the ant, Aphaenogaster rudis. Insect. Soc. 49: 320-325
Boulay R., Fedriani J.M., Manzaneda A.J. and Cerdá X. 2005. Indirect effects of alternative food resources in an ant-plant interaction. Oecologia 144: 72-79
Boulay R., Coll-Toledano J. and Cerdá X. 2006. Geographic variations in Helleborus foetidus elaiosome lipid composition: implications for dispersal by ants Chemoecology 16: 1-7
Boulay R., Carro F., Soriguer R. and Cerdá X. 2007a. Synchrony between fruit maturation and effective disperser’s foraging activity increases seed protection against seed predators. Proc. R. Soc. B 274: 2515-2522
Boulay R., Coll-Toledano J., Manzaneda J.A. and Cerdá X. 2007b. Geographic variations in seed-dispersal by ants: are plant and seed traits decisive? Naturwissenschaften 94: 242-246
Boulay R., Hefetz A., Cerdá X., Devers S., Francke W., Twele R. and Lenoir A. 2007c. Production of sexuals in a fission-performing ant: dual effects of queen pheromones and colony size. Behav. Ecol. Sociobiol. 61: 1531-1541
Boulay R., Carro F., Soriguer R. and Cerdá X. 2009a. Small-scale indirect effects determine the outcome of a tripartite plant-disperser-granivore interaction. Oecologia 161: 529-537
Boulay R., Cerdá X., Fertin A., Ichinose K. and Lenoir A. 2009b. Brood development into sexual females depends on the presence of a queen but not on temperature in an ant dispersing by colony fission, Aphaenogaster senilis. Ecol. Entomol. 34: 595-602
Brew C.R., O’Dowd D.J. and Rae I.D. 1989. Seed dispersal by ants: behaviour-releasing compounds in elaiosomes. Oecologia 80: 490-497
Bronstein J.L. 1994. Our current understanding of mutualism. Q. Rev. Biol. 69: 31-51
Caut S., Barroso A., Amor F. Cerdá X. and Boulay R. in press. A year in an ant’s life: opportunism and seasonal variation in the foraging ecology of Aphaenogaster senilis Ecoscience
Cerdá X., Angulo E., Boulay R. and Lenoir A. 2009. Individual and collective foraging decisions: a field study of worker recruitment in the gypsy ant Aphaenogaster senilis. Behav. Ecol. Sociobiol. 63: 551-562
Christianini A.V., Mayhé-Nunes A.J. and Oliveira P.S. 2007. The role of ants in the removal of non-myrmecochorous diaspores and seed germination in a Neotropical savanna. J. Trop. Ecol. 23: 343-351
Christianini A.V. and Oliveira P.S. 2009. The relevance of ants as seed rescuers of a primarily bird-dispersed tree in the Neotropical cerrado savanna. Oecologia 160: 735-745
Christianin V. and Oliveira P.S. 2010. Birds and ants provide complementary seed dispersal in a neotropical savanna J. Ecol. 98: 573-582
Debussche M., Cortez J. and Rimbault I. 1987. Variation in fleshy fruit composition in the Mediterranean region: the importance of ripening season, life-form, fruit type and geo-graphical distribution. Oikos 49: 244-252
Eisner T. 1957. A comparative morphological study of the proventriculus of ants (Hymenoptera: Formicidae). Bull. Mus. Comp. Zool. Harv. Coll. 116: 439-490
Espadaler X. and Gómez C. 1996. Seed production, predation and dispersal in the Mediterranean myrmecochore Euphorbia characias (Euphorbiaceae). Ecography 19: 7-15
Espadaler X. and Gómez C. 1997. Soil surface searching and transport of Euphorbia characias seeds by ants. Acta Oecol. 18: 39-46
Fenner M. and Thompson K. 2004. The Ecology of Seeds. Cambridge University Press, Cambridge
Fischer R.C., Ölzant S.M., Wanek W. and Mayer V. 2005. The fate of Corydalis cava elaiosomes within an ant colony of Myrmica rubra: elaiosomes are preferentially fed to larvae. Insect. Soc. 52: 55-62
Fischer R.C., Richter A., Hadacek F. and Mayer V. 2008. Chemical differences between seeds and elaiosomes indicate an adaptation to nutritional needs of ants Oecologia 155: 539-547
Fokuhl G., Heinze J. and Poschlod P. 2007. Colony growth in Myrmica rubra with supplementation of myrmecochorous seeds Ecol. Res. 22: 845-847
Fokuhl G., Heinze J. and Poschlod P. 2012. Myrmecochory by small ants - Beneficial effects through elaiosome nutrition and seed dispersal. Acta Oecol. 38: 71-76
Fourcassié V. and Oliveira P.S. 2002. Foraging ecology of the giant Amazonian ant Dinoponera gigantea (Hymenoptera, Formicidae, Ponerinae): activity schedule, diet and spatial foraging patterns. J. Nat. Hist. 18: 2211-2227
Gammans N., Bullock J.M. and Schönrogge K. 2005. Ant benefits in a seed dispersal mutualism. Oecologia 146: 43-49
Giladi I. 2006. Choosing benefits or partners: a review of the evidence for the evolution of myrmecochory. Oikos 112: 481-492
Gorb E. and Gorb S. 2003. Seed Dispersal by Ants in a Deciduous Forest Ecosystem. Kluwer, Dordrecht
Gove A.D., Majer J.D. and Dunn R.R. 2007. A keystone ant species promotes seed dispersal in a “diffuse” mutualism. Oecologia 153: 687-697
Grooms L. 2006. The pros and cons of TZ testing. Seed Today 10: 58-60
Heithaus E.R. 1981. Seed predation by rodents on 3 ant-dispersed plants. Ecology 62: 136-145
Herrera C.M. 1989. Frugivory and seed dispersal by carnivorous mammals and associated fruit characteristics, in undisturbed Mediterranean habitats. Oikos 55: 250-262
Herrera C.M. 1995. Plant-vertebrate seed dispersal systems in the Mediterranean: ecological, evolutionary, and historical determinants. Annu. Rev. Ecol. Syst. 26: 705-727
Herrera C.M. 2001. Dispersión de semillas por animales en el Mediterráneo: ecología y evolución. In: Ecosistemas mediterráneos. Análisis funcional (Zamora R. and Pugnaire F.I., Eds). CSIC - Asociación Española de Ecología. Madrid, Spain, pp 125-152
Herrera C.M. 2002. Seed dispersal by vertebrates. In: Plant–Animal Interactions (Herrera C.M. and Pellmyr O., Eds). Blackwell, Oxford, pp 185-208
Horvitz C.C. and Schemske D.W. 1986. Seed dispersal of a Neotropical myrmecochore: variation in removal rates and dispersal distance. Biotropica 18: 319-323
Hughes L. and Westoby M. 1992. Fate of seeds adapted for dispersal by ants in Australian sclerophyll vegetation. Ecology 73: 1285-1299
Hughes L., Westoby M. and Jurado E. 1994. Convergence of elaiosomes and insect prey: evidence from ant foraging behavior and fatty acid composition. Funct. Ecol. 8: 358-365
Hulme P. 1997. Post-dispersal seed predation and the establishment of vertebrate dispersed plants in Mediterranean scrublands. Oecologia 111: 91-98
Janzen D.H. 1983. Seed and pollen dispersal by animals: convergence in the ecology of contamination and sloppy harvest. Biol. J. Linn. Soc. 20: 103-113
Jordano P. 1989. Pre-dispersal biology of Pistacia lentiscus (Anacardiaceae): cumulative effects on seeds removal by birds. Oikos 55: 375-386
Jordano P. 1995. Angiosperm fleshy fruits and seed dispersers: a comparative analysis of adaptation and constraints in plant–animal interactions. Am. Nat. 145: 163-191
Lubertazzi D., Lubertazzi M.A.A., McCoy N., Gove A.D., Majer J.D. and Dunn R.R. 2010. The ecology of a keystone seed disperser, the ant Rhytidoponera violacea. J. Insect. Sc. 10: 158
Manzaneda A.J., Rey P. and Boulay R. 2007. Geographic and temporal variations in the ant–seed dispersal assemblage of the perennial herb Helleborus foetidus. Biol. J. Linn. Soc. 92: 135-150
Manzaneda A.J. and Rey P.J. 2012. Geographical and interspecific variation and the nutrient-enrichment hypothesis as an adaptive advantage of myrmecochory. Ecography 35: 322-332
Marussich W.A. 2006. Testing myrmecochory from the ant’s perspective: The effects of Datura wrightii and D. discolor on queen survival and brood production in Pogonomyrmex californicus. Insect. Soc. 53: 403-411
McKey D. 1975. The ecology of coevolved seed dispersal systems. In: Coevolution of Animals and Plants (Gilbert L.E. and Raven P.H., Eds). University of Texas Press, Austin, USA. pp 159-191
Méndez M. and Díaz A. 2001. Flowering dynamics in Arum italicum (Araceae): relative role of inflorescence traits, flowering synchrony, and pollination context on fruit initiation Am. J. Bot. 88: 1774-1780
Morales M.A. and Heithaus E.R. 1998. Food from seed-dispersal mutualism shifts sex ratios in colonies of the ant Aphaenogaster rudis. Ecology 79: 734-739
Ness J.H., Morin D.F. and Giladi I. 2009. Uncommon specialization in a mutualism between a temperate herbaceous plant guild and an ant: are Aphaenogaster ants keystone mutualists? Oikos 118: 1793-1804
Passos L. and Oliveira P.S. 2004. Interaction between ants and fruits of Guapira opposita (Nyctaginaceae) in a Brazilian sandy plain rainforest: ant effects on seeds and seedlings. Oecologia 139: 376-382
Pfeiffer M., Huttenlocher H. and Ayasse M. 2010. Myrmecochorous plants use chemical mimicry to cheat seed-dispersing ants. Funct. Ecol. 24: 545-555
Pizo M.A. and Oliveira P.S. 1998. Interactions between ants and seeds of a non-myrmecochorous neotropical tree, Cabralea canjerana (Meliaceae), in the atlantic forest of southeast Brazil. Am. J. Bot. 85: 669-674
Pizo M.A. and Oliveira P.S. 2000. The use of fruits and seeds by ants in the Atlantic forest of southeast Brazil. Biotropica 32: 851-861
R Development Core Team 2010. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Roberts J.T. and Heithaus E.R. 1986. Ants rearrange the vertebrate-generated seed shadow of a neotropical fig tree. Ecology 67: 1046-1051
Snow D.W. 1971. Evolutionary aspects of fruit-eating by birds. Ibis 113: 194-202
Traveset A. 1994. Cumulative effects on the reproductive output of Pistacia terebinthus (Anacardiaceae). Oikos 71: 152-162
Wang B.C. and Smith T.B. 2002. Closing the seed dispersal loop. Trends Ecol. Evol. 17: 379-385
Wheelwright N.T. and Orians G.H. 1982. Seed dispersal by animals: contrasts with pollen dispersal, problems of terminology, and constraints on coevolution. Am. Nat. 119: 4020-413
Zelikova T.J., Dunn R.R. and Sanders N.J. 2008. Variation in seed dispersal along an elevational gradient in Great Smoky Mountains National Park. Acta Oecol. 34: 155-162
Acknowledgments
We are grateful to Ana Carvajal for assistance in the laboratory and Dr Marcos Méndez for information on the biology of Arum italicum. Jacqueline Minett helped editing English. We are also grateful to Dr Elena Angulo, Prof Johan Billen and two anonymous reviewers for greatly improving the manuscript. This work was funded by MICINN and FEDER (projects CGL2009-12472 to RB and CGL2009-09690 to XC) and MICINN (project CONSOLIDER-MONTES CSD2008-00040 to RB and XC); AB thanks the Consejería de Educación (Junta de Andalucía) for work leave. We thank the authorities of Doñana Natural Park for providing permits and facilities to conduct this study. All experiments comply with current Spanish legislation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barroso, Á., Amor, F., Cerdá, X. et al. Dispersal of non-myrmecochorous plants by a “keystone disperser” ant in a Mediterranean habitat reveals asymmetric interdependence. Insect. Soc. 60, 75–86 (2013). https://doi.org/10.1007/s00040-012-0268-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-012-0268-0