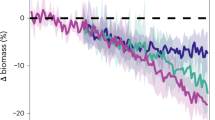
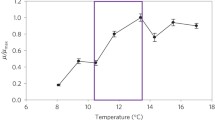
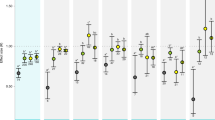
Abstract
Climate change impacts prevail on marine pelagic systems and food webs, including zooplankton, the key link between primary producers and fish. Several metabolic, physiological, and ecological responses of zooplankton species and communities to global stressors have recently been tested, with an emerging field in assessing effects of combined climate-related factors. Yet, integrative studies are needed to understand how ocean acidification interacts with global warming, mediating zooplankton body chemistry and ecology. Here, we tested the combined effects of global warming and ocean acidification, predicted for the year 2100, on a community of calanoid copepods, a ubiquitously important mesozooplankton compartment. Warming combined with tested pCO2 increase affected metabolism, altered stable isotope composition and fatty acid contents, and reduced zooplankton fitness, leading to lower copepodite abundances and decreased body sizes, and ultimately reduced survival. These interactive effects of temperature and acidification indicate that metabolism-driven chemical responses may be the underlying correlates of ecological effects observed in zooplankton communities, and highlight the importance of testing combined stressors with a regression approach when identifying possible effects on higher trophic levels.







Similar content being viewed by others
References
Aksnes DL, Ohman MD (1996) A vertical life table approach to zooplankton mortality estimation. Limnol Oceanogr 41:1461–1469. doi:10.4319/lo.1996.41.7.1461
Anderson TR, Hessen DO, Elser JJ, Urabe J (2005) Metabolic stoichiometry and the fate of excess carbon and nutrients in consumers. Am Nat 165:1–15. doi:10.1086/426598
Bellerby RGJ, Schulz KG, Riebesell U et al (2008) Marine ecosystem community carbon and nutrient uptake stoichiometry under varying ocean acidification during the PeECE III experiment. Biogeosciences 5:1517–1527
Bi R, Ismar S, Sommer U, Zhao M (2016) Environmental dependence of the correlations between stoichiometric and fatty acid-based indicators of phytoplankton nutritional quality. Limnol Oceanogr 62:334–347. doi:10.1002/lno.10429
Boersma M (2000) The nutritional quality of P-limited algae for Daphnia. Limnol Oceanogr 45:1157–1161
Boersma M, Mathew KA, Niehoff B et al (2016) Temperature driven changes in the diet preference of omnivorous copepods: no more meat when it's hot? Ecol Lett 19:45–53. doi:10.1111/ele.12541
Bolker BM, Brooks ME, Clark CJ et al (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135. doi:10.1016/j.tree.2008.10.008
Boyce DG, Lewis MR, Worm B (2010) Global phytoplankton decline over the past century. Nature 466:591–596. doi:10.1038/nature09268
Brett MT, Müller-Navarra DC (1997) The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshwater Biol 38:483–499
Brock TD (1981) Calculating solar-radiation for ecological-studies. Ecol Model 14:1–19. doi:10.1016/0304-3800(81)90011-9
Brown JH, Gillooly JF, Allen AP et al (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789
Byrne M, Przeslawski R (2013) Multistressor impacts of warming and acidification of the ocean on marine invertebrates’ life histories. Integr Comp Biol 53:582–596. doi:10.1093/icb/ict049
Cohen J (1992) Statistical power analysis. Curr Dir Psychol Sci 1(3):98–101
Cottingham KL, Lennon JT, Brown BL (2005) Knowing when to draw the line: designing more informative ecological experiments. Front Ecol Environ 3:145–152. doi:10.1890/1540-9295(2005)003[0145:KWTDTL]2.0.CO;2
Crain CM, Kroeker K, Halpern BS (2008) Interactive and cumulative effects of multiple human stressors in marine systems. Ecol Lett 11:1304–1315. doi:10.1111/j.1461-0248.2008.01253.x
Cripps G, Lindeque P, Flynn K (2014) Parental exposure to elevated pCO2 influences the reproductive success of copepods. J Plankton Res 36:1165–1174. doi:10.1093/plankt/fbu052
Cripps G, Flynn KJ, Lindeque PK (2016) Ocean acidification affects the phyto-zoo plankton trophic transfer efficiency. PLoS ONE 11(4):e0151739. doi:10.1371/journal.pone.0151739
Darchambeau F, Faerovig PJ, Hessen DO (2003) How Daphnia copes with excess carbon in its food. Oecologia 136:336–346. doi:10.1007/s00442-003-1283-7
Daufresne M, Lengfellner K, Sommer U (2009) Global warming benefits the small in aquatic ecosystems. PNAS 106:12788–12793. doi:10.1073/pnas.0902080106
De Wit P, Dupont S, Thor P (2015) Selection on oxidative phosphorylation and ribosomal structure as a multigenerational response to ocean acidification in the common copepod Pseudocalanus acuspes. Evol Appl. doi:10.1111/eva.12335
Dell AI, Pawar S, Savage VM (2011) Systematic variation in the temperature dependence of physiological and ecological traits. PNAS 108:10591–10596. doi:10.1073/pnas.1015178108
DeMott WR, Tessier AJ (2002) Stoichiometric constraints vs. algal defenses: testing mechanisms of zooplankton food limitations. Ecology 83:3426–3433
Doney SC, Ruckelshaus M, Duffy JE et al (2012) Climate change impacts on marine ecosystems. In: Carlson CA, Giovannoni SJ (eds) Annual review of marine science, vol 4. Annual Reviews, Palo Alto, pp 11–37
Dupont S, Thorndyke MC (2009) Impact of CO2-driven ocean acidification on invertebrates early life-history—what we know, what we need to know and what we can do. Biogeosciences 6:3109–3131
Edwards M, Richardson AJ (2004) Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430:881–884
Eiane K, Ohman MD (2004) Stage-specific mortality of Calanus finmarchicus, Pseudocalanus elongatus and Oithona similis on Fladen Ground, North Sea, during a spring bloom. Mar Ecol Prog Ser 268:183–193
Evjemo JO, Tokle N, Vadstein O, Olsen Y (2008) Effect of essential dietary fatty acids on egg production and hatching success of the marine copepod Temora longicornis. J Exp Mar Biol Ecol 365:31–37. doi:10.1016/j.jembe.2008.07.032
Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65:414–432. doi:10.1093/icesjms/fsn048
Filstrup CT, Hillebrand H, Heathcote AJ et al (2014) Cyanobacteria dominance influences resource use efficiency and community turnover in phytoplankton and zooplankton communities. Ecol Lett 17:464–474. doi:10.1111/ele.12246
Fitzer SC, Caldwell GS, Close AJ, Clare AS (2012) Ocean acidification induces multi-generational decline in copepod naupliar production with possible conflict for reproductive resource allocation. J Exp Mar Biol Ecol 418–419:30–36. doi:10.1016/j.jembe.2012.03.009
Garzke J, Ismar SMH, Sommer U (2015) Climate change affects low trophic level marine consumers: warming decreases copepod size and abundance. Oecologia 177:849–860. doi:10.1007/s00442-014-3130-4
Garzke J, Hansen T, Ismar SMH, Sommer U (2016) Combined effects of ocean warming and acidification on copepod abundance, body size and fatty acid content. PLoS One 11:e0155952. doi:10.1371/journal.pone.0155952
Hansen T, Sommer U (2007) Increasing the sensitivity of δ13 C and δ15 N abundance measurements by a high sensitivity elemental analyzer connected to an isotope ratio mass spectrometer. Rapid Commun Mass Sp 21:314–318. doi:10.1002/rcm.2847
Havenhand J, Dupont S, Quinn GP (2010) Designing ocean acidification experiments to maximise inference. In: Riebesell U, Fabry VJ, Hansson L, Gattuso JP (eds) Guide to best practices for ocean acidification research and data reporting. Publications Office of the European Union, Brussels, Luxembourg
Hildebrandt N, Niehoff B, Sartoris FJ (2014) Long-term effects of elevated CO2 and temperature on the Arctic calanoid copepods Calanus glacialis and C. hyperboreus. Mar Pollut Bull 80:59–70. doi:10.1016/j.marpolbul.2014.01.050
Hirche H-J, Niehoff B (1996) Reproduction of the Arctic copepod Calanus hyperboreus in the Greenland Sea-field and laboratory observations. Polar Biol 16:209–219. doi:10.1007/BF02329209
Holste L, Peck MA (2005) The effects of temperature and salinity on egg production and hatching success of Baltic Acartia tonsa (Copepoda: Calanoida): a laboratory investigation. Mar Biol 148:1061–1070. doi:10.1007/s00227-005-0132-0
Ikeda T, Kanno Y, Ozaki K, Shinada A (2001) Metabolic rates of epipelagic marine copepods as a function of body mass and temperature. Mar Biol 139:587–596
IPCC (2014) Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
Kordas RL, Harley CDG, O’Connor MI (2011) Community ecology in a warming world: the influence of temperature on interspecific interactions in marine systems. J Exp Mar Biol Ecol 400:218–226. doi:10.1016/j.jembe.2011.02.029
Kurihara H (2008) Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar Ecol Prog Ser 373:275–284. doi:10.3354/meps07802
Kurihara H, Ishimatsu A (2008) Effects of high CO2 seawater on the copepod (Acartia tsuensis) through all life stages and subsequent generations. Mar Pollut Bull 56:1086–1090. doi:10.1016/j.marpolbul.2008.03.023
Kurihara H, Shimode S, Shirayama Y (2004) Effects of raised CO2 concentration on the egg production rate and early development of two marine copepods (Acartia steueri and Acartia erythraea). Mar Pollut Bull 49:721–727. doi:10.1016/j.marpolbul.2004.05.005
Leandro SM, Queiroga H, Rodriguez-Grana L, Tiselius P (2006) Temperature-dependent development and somatic growth in two allopatric populations of Acartia clausi (Copepoda : Calanoida). Mar Ecol Prog Ser 322:189–197
Lennartz ST, Lehmann A, Herrford J et al (2014) Long-term trends at the Boknis Eck time series station (Baltic Sea), 1957–2013: does climate change counteract the decline in eutrophication? Biogeosciences 11:6323–6339. doi:10.5194/bg-11-6323-2014
Longhurst AR (1985) The structure and evolution of plankton communities. Prog Oceanogr 15:1–35. doi:10.1016/0079-6611(85)90036-9
Mackas DL, Greve W, Edwards M et al (2012) Changing zooplankton seasonality in a changing ocean: Comparing time series of zooplankton phenology. Prog Oceanogr 97:31–62
Malzahn AM, Boersma M (2012) Effects of poor food quality on copepod growth are dose dependent and non-reversible. Oikos 121:1408–1416. doi:10.1111/j.1600-0706.2011.20186.x
Malzahn AM, Aberle N, Clemmensen C, Boersma M (2007) Nutrient limitation of primary producers affects planktivorous fish condition. Limnol Oceanogr 52:2062–2071
Malzahn AM, Hantzsche F, Schoo KL et al (2010) Differential effects of nutrient-limited primary production on primary, secondary or tertiary consumers. Oecologia 162:35–48. doi:10.1007/s00442-009-1458-y
Mayor DJ, Anderson TR, Pond DW, Irigoien X (2009) Egg production and associated losses of carbon, nitrogen and fatty acids from maternal biomass in Calanus finmarchicus before the spring bloom. J Mar Sys 78:505–510. doi:10.1016/j.jmarsys.2008.12.019
Mayor DJ, Everett NR, Cook KB (2012) End of century ocean warming and acidification effects on reproductive success in a temperate marine copepod. J Plankton Res 34:258–262. doi:10.1093/plankt/fbr107
Mayor DJ, Sommer U, Cook KB, Viant MR (2015) The metabolic response of marine copepods to environmental warming and ocean acidification in the absence of food. Sci Rep. doi:10.1038/srep13690
Möllmann C, Kornilovs G, Fetter M, Köster FW (2005) Climate, zooplankton, and pelagic fish growth in the central Baltic Sea. ICES J Mar Sci 62:1270–1280
Müller-Navarra DC (2008) Food web paradigms: the biochemical view on trophic interactions. Int Rev Hydrobiol 93:489–505. doi:10.1002/iroh.200711046
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42. doi:10.1038/nature01286
Paul C, Matthiessen B, Sommer U (2015) Warming but not enhanced CO2 quantitatively and qualitatively affects phytoplankton biomass. Mar Ecol Prog Ser. doi:10.3354/meps11264
Paul C, Sommer U, Garzke J et al (2016) Effects of increased CO2 concentration on nutrient limited coastal summer plankton depend on temperature. Limnol Oceanogr 61:853–868. doi:10.1002/lno.10256
Pedersen SA, Hansen BH, Altin D, Olsen AJ (2013) Medium-term exposure of the North Atlantic copepod Calanus finmarchicus (Gunnerus, 1770) to CO2-acidified seawater: effects on survival and development. Biogeosciences 10:7481–7491. doi:10.5194/bg-10-7481-2013
Pedersen SA, Håkedal OJ, Salaberria I et al (2014) Multigenerational exposure to ocean acidification during food limitation reveals consequences for copepod scope for growth and vital rates. Environ Sci Technol 48:12275–12284. doi:10.1021/es501581j
Pierrot D, Lewis E, Wallace D (2006) MS Excel program developed for CO2 system calculations: ORNL/CDIAC-105a. Carbon Dioxide Information Analysis Centre Oak Ridge National Laboratory, US Department of Energy, Oak Rodge, Tennessee
Plath K, Boersma M (2001) Mineral limitation of zooplankton: stoichiometric constraints and optimal foraging. Ecology 82:1260–1269. doi:10.1890/0012-9658(2001)082[1260:mlozsc]2.0.co;2
Pörtner HO, Farrell AP (2008) Ecology: physiology and climate change. Science 322:690–692. doi:10.1126/science.1163156
Pörtner HO, Langenbuch M, Reipschläger A (2004) Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J Oceanogr 60:705–718. doi:10.1007/s10872-004-5763-0
Przeslawski R, Byrne M, Mellin C (2015) A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob Change Biol 21:2122–2140. doi:10.1111/gcb.12833
Rice E, Dam HG, Stewart G (2014) Impact of climate change on estuarine zooplankton: surface water warming in long island sound is associated with changes in copepod size and community structure. Estuar Coasts. doi:10.1007/s12237-014-9770-0
Richardson AJ (2004) Climate impact on plankton ecosystems in the northeast Atlantic. Science 305:1609–1612. doi:10.1126/science.1100958
Richardson AJ (2008) In hot water: zooplankton and climate change. ICES J Mar Sci 65:279–295. doi:10.1093/icesjms/fsn028
Schoo KL, Malzahn AM, Krause E, Boersma M (2013) Increased carbon dioxide availability alters phytoplankton stoichiometry and affects carbon cycling and growth of a marine planktonic herbivore. Mar Biol 160:2145–2155. doi:10.1007/s00227-012-2121-4
Schulz KG, Bellerby RGJ, Brussaard CPD et al (2013) Temporal biomass dynamics of an Arctic plankton bloom in response to increasing levels of atmospheric carbon dioxide. Biogeosciences 10:161–180. doi:10.5194/bg-10-161-2013
Sommer U, Sommer F (2005) Cladocerans versus copepods: the cause of contrasting top–down controls on freshwater and marine phytoplankton. Oecologia 147:183–194. doi:10.1007/s00442-005-0320-0
Speekmann CL, Hyatt CJ, Buskey EJ (2006a) Effects of Karenia brevis diet on RNA:DNA ratios and egg production of Acartia tonsa. Harmful Algae 5:693–704. doi:10.1016/j.hal.2006.03.002
Speekmann CL, Nunez BS, Buskey EJ (2006b) Measuring RNA:DNA ratios in individual Acartia tonsa (Copepoda). Mar Biol 151:759–766. doi:10.1007/s00227-006-0520-0
Sterner RW (2010) Role of Zooplankton in Aquatic Ecosystems. In: Likens GE (ed) Encyclopedia of inland waters, 1st edn. Elsevier, Oxford, UK, pp 678–688
Sterner RW, Elser JJ (2002) Ecological stochiometry: the biology of elements from molecules to the biosphere. Princeton University Press, New Jersey
Stocker TF, Quin D, Plattner GH et al (2013) IPCC, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom
Thomsen J, Gutowska MA, Saphorster J et al (2010) Calcifying invertebrates succeed in a naturally CO2-rich coastal habitat but are threatened by high levels of future acidification. Biogeosciences 7:3879–3891. doi:10.5194/bg-7-3879-2010
Thor P, Dupont S (2015) Transgenerational effects alleviate severe fecundity loss during ocean acidification in a ubiquitous planktonic copepod. Glob Change Biol 21:2261–2271. doi:10.1111/gcb.12815
Urabe J, Togari JUN, Elser JJ (2003) Stoichiometric impacts of increased carbon dioxide on a planktonic herbivore. Glob Change Biol 9:818–825. doi:10.1046/j.1365-2486.2003.00634.x
Vehmaa A, Brutemark A, Engstrom-Ost J (2012) Maternal effects may act as an adaptation mechanism for copepods facing ph and temperature changes. PLoS One. doi:10.1371/journal.pone.0048538
Vehmaa A, Almén AK, Brutemark A (2015) Ocean acidification challenges copepod reproductive plasticity. Biogeosciences 12:18541–18570
Whiteley NM (2011) Physiological and ecological responses of crustaceans to ocean acidification. Mar Ecol Prog Ser 430:257–271. doi:10.3354/meps09185
Zervoudaki S, Frangoulis C, Giannoudi L, Krasakopoulou E (2014) Effects of low pH and raised temperature on egg production, hatching and metabolic rates of a Mediterranean copepod species (Acartia clausi) under oligotrophic conditions. Mediterr Mar Sci 15:74–83
Zhang D, Li S, Wang G, Guo D (2011) Impacts of CO2-driven seawater acidification on survival, egg production rate and hatching success of four marine copepods. Acta Oceanol Sin 30:86–94. doi:10.1007/s13131-011-0165-9
Acknowledgements
The authors thank the BMBF (German Ministry of Education and Research) for funding the project BIOACID II. We especially thank A. Paul, C. Paul, H. Horn, M. Rathmer, D. Riemann, M. Rönspies and L. Gobelius for assistance in the installation of the mesocosm facility, sampling and sample preparation. T. Hansen is thanked for great technical support. A. Ludwig is acknowledged for analyzing DIC and Ashlie Maack for language editing. We thank Stuart Findlay and three anonymous referees for helpful review of our work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Garzke, J., Sommer, U. & Ismar, S.M.H. Is the chemical composition of biomass the agent by which ocean acidification influences on zooplankton ecology?. Aquat Sci 79, 733–748 (2017). https://doi.org/10.1007/s00027-017-0532-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-017-0532-5