Abstract
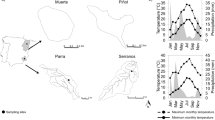
Among the environmental factors affecting benthic algae and cyanobacteria in streams, the one often producing the largest effects is flow intermittency. This study aimed to characterize the responses of algal assemblages to flow intermittency in a Mediterranean intermittent stream during the drying, non-flow (112 days), and rewetting phases. Algae growing in the epilithic, epipsammic and hyporheic streambed compartments were analyzed for pigment composition, and for the existence of structural changes in cells. Chlorophyll-a concentrations decreased between 60 to 90 % during the non-flow phase, indicating low resistance of algal assemblages to desiccation. In contrast, fast recoveries of Chlorophyll-a when flow resumed indicated high resilience. Pigment composition revealed that the epilithic algal assemblage was considerably different than the epipsammic and hyporheic ones. These differences were mainly attributed to the physical conditions prevailing on each streambed compartment that allowed the growth of different algal assemblages. During the non-flow phase, the synthesis of protective carotenoids (i.e. echinenone and scytonemin) and the occurrence of cell resistance structures (i.e. enlarged membrane thickness and resistant spores) enhanced resistance of the epilithic biofilm. The resistance observed in the epilithic biofilm might also be related to the tightly adhered growth-form of algae on this substratum. Main results suggest that algal assemblages in the epilithic compartment, which were the most exposed to desiccation, were structurally and functionally better adapted to flow interruption than those colonizing other streambed compartments, and that this compartment plays a crucial role in maintaining ecosystem functions under varying flow periods.





Similar content being viewed by others
References
Acuña V, Giorgi A, Muñoz I, Uehlinger U, Sabater S (2004) Flow extremes and benthic organic matter shape the metabolism of a headwater Mediterranean stream. Freshw Biol 49:960–971
Adams WW, Demmig-Adams B, Lange OL (1993) Carotenoid composition and metabolism in green and blue-green algal lichens in the field. Oecologia 94:576–584
Agrawal SC, Singh V (2000) Vegetative survival, akinete formation and germination in three blue-green algae and one green alga in relation to light intensity, temperature, heat shock and UV exposure. Folia Microbiol (Praha) 45:439–446
Angradi TR, Kubly DM (1993) Effects of atmospheric exposure on chlorophyll-a, biomass and productivity of the epilithon of a tailwater river. Regul Rivers Res Manag 8:345–358
Armstrong GA, Hearst JE (1996) Genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J 10:228–237
Belnap J, Lange OL (2003) Structure and functioning of biological soil crusts: a synthesis. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function and management. Springer, Heidelberg, pp 471–479
Belnap J, Phillips SL, Smith SD (2007) Dynamics of cover, UV-protective pigments, and quantum yield in biological soil crust communities of an undisturbed Mojave Desert shrubland. Flora 202:674–686
Bergey EA (2005) How protective are refuges? Quantifying algal protection in rock crevices. Freshw Biol 50:1163–1177
Bergey EA, Bunlue P, Silalom S, Thapanya D, Chantaramongkol P (2010) Environmental and biological factors affect desiccation tolerance of algae from two rivers (Thailand and New Zealand) with fluctuating flow. J N Am Benthol Soc 29:725–736
Bidigare R, van Heukelem L, Trees CC (2005) Analysis of algal pigments by high-performance liquid chromatography. In: Andersen RA (ed) Algal culturing techniques. Elsevier Academic Press, Burlington, pp 327–345
Billi D, Potts M (2002) Life and death of dried prokaryotes. Res Microbiol 153:7–12
Blinn DW, Shannon JP, Stevens LE, Carder JP (1995) Consequences of fluctuating discharge for lotic communities. J N Am Benthol Soc 14:233–248
Buchaca T (2009) Pigments indicadors: estudi del senyal en estanys dels Pirineus i de la seva aplicació en paleolimnologia. Arxius de les Seccions de Ciències; 142. Institut d’Estudis Catalans
Buchaca T, Catalan J (2007) Factors influencing the variability of pigments in the surface sediments of mountain lakes. Freshw Biol 52:1365–1379
Burkholder JM (1996) Interactions of benthic algae with their substrata. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, San Diego, pp 253–297
Butturini A, Alvarez M, Bernal S, Vazquez E (2008) Diversity and temporal sequences of forms of DOC and NO−3 discharge responses in an intermittent stream: predictable or random succession? J Geophys Res 113:1–10
Caramujo MJ, Mendes CRB, Cartaxana P, Brotas V, Boavida MJ (2008) Influence of drought on algal biofilms and meiofaunal assemblages of temperate reservoirs and rivers. Hydrobiologia 598:77–94
Collyer DM, Fogg GE (1955) Studies on fat accumulation by algae. J Exp Bot 6:256–275
Corcoll N, Bonet B, Morin S, Tlili A, Leira M, Guasch H (2012) The effect of metals on photosynthesis processes and diatom metrics of biofilm from a metal-contaminated river: a translocation experiment. Ecol Indic 18:620–631
Daley RJ (1973) Experimental characterization of lacustrine chlorophyll diagenesis: II. Bacterial, viral and herbivore grazing effects. Arch Hydrobiol 72(4):409–439
Davies BH (1976) Carotenoids. Academic Press, London
Davis JS (1972) Survival records in the algae, and the survival role of certain algal pigments, fat, and mucilaginous substances. Biologist 54:52–93
Dieser M, Greenwood M, Foreman CM (2010) Carotenoid pigmentation in Antarctic heterotrophic bacteria as a strategy to withstand environmental stresses. Arct Antarct Alp Res 42:396–405
Döll P, Schmied HM (2012) How is the impact of climate change on river flow regimes related to the impact on mean annual runoff? A global-scale analysis. Environ Res Lett 7:1–11
Dorigo U, Bourrain X, Bérard A, Leboulanger C (2004) Seasonal changes in the sensitivity of river microalgae to atrazine and isoproturon along a contamination gradient. Sci Total Environ 318:101–114
Evans JH (1958) The survival of freshwater algae during dry periods: part I. An investigation of the algae of five small ponds. J Ecol 46:149–167
Evans JH (1959) The survival of freshwater algae during dry periods: part II. Drying experiments. Part III: stratification of algae in pond margin litter and mud. J Ecol 47:55–81
Fernández-Valiente E, Camacho A, Rochera C, Rico E, Vincent WF, Quesada A (2007) Community structure and physiological characterization of microbial mats in Byers Peninsula, Livingston Island (South Shetland Islands, Antarctica). FEMS Microbiol Ecol 59:377–385
Findlay S (1995) Importance of surface-subsurface exchange in stream ecosystem: the hyporheic zone. Limnol Oceanogr 40:159–164
Fleming ED, Castenholz RW (2007) Effects of periodic desiccation on the synthesis of the UV-screening compound, scytonemin, in cyanobacteria. Environ Microbiol 9:1448–1455
Garcia-Pichel F, Castenholz RW (1991) Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. J Phycol 27:395–409
Haney PJ, Badger JH, Buldak GL et al (1999) Thermal adaptation analyzed by comparison of protein sequences from mesophilic and extremely thermophilic Methanococcus species. Proc Natl Acad Sci 96:3578–3583
Hawes I, Howard-Williams C, Vincent W (1992) Desiccation and recovery of Antarctic cyanobacterial mats. Polar Biol 12:587–594
Hill MO, Gauch HG (1980) Detrended correspondence analysis: an improved ordination technique. Vegetatio 42:47–58
Hills DR, Hladuns SL, Scherero S, Potts M (1994) Water stress proteins of Nostoc commune (Cyanobacteria) are secreted with UV-A/B absorbing pigments and associate with 1,4-β-D-xylanxylanohydrolase activity. J Biol Chem 269:7726–7734
Hirabayashi Y, Shinjiro K, Emori S, Taikan O, Masahide K (2008) Global projections of changing risks of floods and droughts in a changing climate. Hydrol Sci J 53:754–772
Holzinger A, Karsten U (2013) Desiccation stress and tolerance in green algae: consequences for ultrastructure, physiological and molecular mechanisms. Front Plant Sci 4:327
Hostetter HP, Hoshaw RW (1970) Environmental factors affecting resistance to desiccation in the diatom Stauroneis anceps. Am J Bot 57:517–518
Jeffrey SW, Mantoura RFC, Wright SW (1997) Phytoplankton pigments in oceanography: guidelines to modern methods. UNESCO, Paris
Karsten U, Maier J, Garcia-Pichel F (1998) Seasonality in UV-absorbing compounds of cyanobacterial mat communities from an intertidal mangrove flat. Aquat Microb Ecol 16:37–44
Lake PS (2003) Ecological effects of perturbation by drought in flowing waters. Freshw Biol 48:1161–1172
Lake PS (2013) Resistance, resilience and restoration. Ecol Manag Restor 14:20–24
Lamberti GA, Resh VH (1985) Comparability of introduced tiles and natural substrates for sampling lotic bacteria, algae and macroinvertebrates. Freshw Biol 15:21–30
Larned S, Datry T, Arscott DB, Tockner K (2010) Emerging concepts in temporary river ecology. Freshw Biol 55:717–738
Ledger ME, Harris RML, Armitage PD, Milner AM (2008) Disturbance frequency influences patch dynamics in stream benthic algal communities. Oecologia 155:809–819
Mantoura RFC, Llewellyn CA (1983) The rapid determination of algal chlorophyll and carotenoid pigments and their breakdown products in natural waters by reverse-phase high-performance liquid chromatography. Anal Chem Acta 151:297–314
Mayer MS, Likens GE (1987) The importance of algae in a shaded headwater stream as food for an abundant caddisfly (Trichoptera). J N Am Benthol Soc 6(4):262–269
McKnight DM, Tate CM, Andrews ED, Niyogi DK, Cozzetto K, Welch K, Lyons WB, Capone DG (2007) Reactivation of a cryptobiotic stream ecosystem in the McMurdo dry valleys, Antarctica: a long-term geomorphological experiment. Geomorphology 89:186–204
Medici C, Bernal S, Butturini A, Sabater F, Wade AJ, Frances F (2010) Modelling the inorganic nitrogen behaviour in a small Mediterranean forested catchment, Fuirosos (Catalonia). Hydrol Earth Syst Sci, pp 5665–5703 (Discuss. 6)
Millie DF, Paerl HW, Hurley JP (1993) Microalgal pigment assessments using high-performance liquid chromatography: a synopsis of organismal and ecological applications. Can J Fish Aquat Sci 50:2513–2527
Milly PCD, Dunne KA, Vecchia AV (2005) Global pattern of trends in stream flow and water availability in a changing climate. Nature 438:347–350
Morison MO, Sheath RG (1985) Responses to desiccation stress by Klebsormidium rivulare (Ulotrichales, Chlorophyta) from a Rhode Island stream. Phycologia 24:129–145
Moss B (1968) Studies on the degradation of chlorophyll a and carotenoids in freshwaters. New Phytol 67:49–59
Murdock JN, Dodds WK (2007) Linking benthic algal biomass to stream substratum topography. J Phycol 43:449–460
Oren A, Gunde-Cimerman N (2007) Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol Lett 269:1–10
Peterson GC (1996) Response of algae to natural physical disturbances. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, San Diego, pp 375–403
Rabbani S, Beyer P, Von Lintig J, Hugueney P, Kleinig H (1998) Induced beta-carotene synthesis driven by triacylglycerol deposition in the unicellular alga Dunaliella bardawil. Plant Physiol 116:1239–1248
Robson BJ (2000) Role of residual biofilm in the recolonization of rocky intermittent streams by benthic algae. Mar Freshw Res 51:725–732
Robson BJ, Matthews TG, Lind PR, Thomas NA (2008) Pathways for algal recolonization in seasonally-flowing streams. Freshw Biol 53:2385–2401
Robson BJ, Chester ET, Mitchell BD, Matthews TG (2013) Disturbance and the role of refuges in mediterranean climate streams. Hydrobiologia 719:77–91
Romani AM, Sabater S (2001) Structure and activity of rock and sand biofilms in a Mediterranean stream. Ecology 82:3232–3245
Romaní AM, Sabater S (1997) Metabolism recovery of a stromatolitic biofilm after drought in a Mediterranean stream. Arch Hydrobiol 140:261–271
Romaní AM, Butturini A, Sabater F, Sabater S (1998) Heterotrophic metabolism in a forest stream sediment: surface versus subsurface zones. Aquat Microb Ecol 16:143–151
Rowan KS (1989) Photosynthetic pigments of algae. Cambridge University Press, Cambridge
Sabater S (2000) Structure and architecture of a stromatolite from a Mediterranean stream. Aquat Microb Ecol 21:161–168
Sabater S, Guasch H, Romaní AM, Muñoz I (2000) Stromatolitic communities in Mediterranean streams: adaptations to a changing environment. Biodiv Conserv 9:379–392
Sabater S, Bernal S, Butturini A, Nin E, Sabater F (2001) Wood and leaf debris input in a Mediterranean stream: the influence of riparian vegetation. Arch Hydrobiol 153:91–102
Sabater S, Elosegi A, Acuña V, Basaguren A, Muñoz I (2008) Effect of climate on the trophic structure of temperate forested streams. A comparison of Mediterranean and Atlantic streams. Sci Total Environ 390:475–484
Solovchenko AE, Khozin-Goldberg I, Cohen Z, Merzlyak MN (2008) Carotenoid-to-chlorophyll ratio as a proxy for assay of total fatty acids and arachidonic acid content in the green microalga Parietochloris incisa. J Appl Phycol 21:361–366
Stanley EH, Fisher SG, Jones JB (2004) Effects of water loss on primary production: a landscape-scale model. Aquat Sci 66:130–138
Stevenson RJ, Bothwell ML, Lowe RL (1996) Algal ecology: freshwater benthic ecosystems. Academic Press, San Diego
Takaichi S (2011) Carotenoids in algae: distributions, biosyntheses and functions. Mar Drugs 9:1101–1118
ter Braak CJF, Smilauer P (2002) CANOCO Reference manual and CanoDraw for Windows user’s guide: software for canonical community ordination (version 4.5), Microcomputer Power, Ithaca. http://www.canoco.com
Timoner X, Acuña V, von Schiller D, Sabater S (2012) Functional responses of stream biofilms to flow cessation, desiccation and rewetting. Freshw Biol 57:1565–1578
Timoner X, Acuña V, Frampton L, Pollard P, Sabater S, Bunn SE (2014) Biofilm functional responses to the rehydration of a dry intermittent stream. Hydrobiologia 727:184–195
Tornés E, Sabater S (2010) Variable discharge alters habitat suitability for benthic algae and cyanobacteria in a forested Mediterranean stream. Mar Freshw Res 61:441–450
Torzillo G, Goksan T, Faraloni C, Kopecky J, Masojídek J (2003) Interplay between photochemical activities and pigment composition in an outdoor culture of Haematococcus pluvialis during the shift from the green to red stage. J Appl Phycol 15:127–136
Vicent WF, Howard-Williams C (1986) Antarctic stream ecosystems: physiological ecology of a blue-green algal epilithon. Freshw Biol 16:219–233
Von Schiller D, Martí E, Riera JL, Ribot M, Argerich A, Fonolla P, Sabater F (2008) Inter-annual, annual, and seasonal variation of P and N retention in a perennial and an intermittent stream. Ecosystems 11:670–687
Wetzel RG (1983) Periphyton of freshwater ecosystems. Dr. W. Junk Publishers, The Hague
Wieners P, Mudimu O, Bilger W (2012) Desiccation-induced non-radiative dissipation in isolated green lichen algae. Photosynth Res 113:239–247
Acknowledgments
We thank Laura Ferrando and Núria Caceres for their help with the HPLC. Xisca Timoner was recipient of a PhD fellowship from the Spanish Ministry of science and technology (AP-2007-01945), and Teresa Buchaca was partially supported by the Spanish Government project Invasive fish (427/2011). This research was funded by the projects SCARCE (CONSOLIDER-INGENIO CSD2009-00065), and CARBONET (CGL2011-30474-C02-01) of the Spanish Ministry of Science and Innovation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Timoner, X., Buchaca, T., Acuña, V. et al. Photosynthetic pigment changes and adaptations in biofilms in response to flow intermittency. Aquat Sci 76, 565–578 (2014). https://doi.org/10.1007/s00027-014-0355-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-014-0355-6