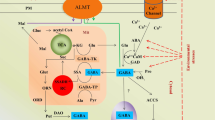
Abstract
The role of γ-aminobutyric acid (GABA) as a signal in animals has been documented for over 60 years. In contrast, evidence that GABA is a signal in plants has only emerged in the last 15 years, and it was not until last year that a mechanism by which this could occur was identified—a plant ‘GABA receptor’ that inhibits anion passage through the aluminium-activated malate transporter family of proteins (ALMTs). ALMTs are multigenic, expressed in different organs and present on different membranes. We propose GABA regulation of ALMT activity could function as a signal that modulates plant growth, development, and stress response. In this review, we compare and contrast the plant ‘GABA receptor’ with mammalian GABAA receptors in terms of their molecular identity, predicted topology, mode of action, and signalling roles. We also explore the implications of the discovery that GABA modulates anion flux in plants, its role in signal transduction for the regulation of plant physiology, and predict the possibility that there are other GABA interaction sites in the N termini of ALMT proteins through in silico evolutionary coupling analysis; we also explore the potential interactions between GABA and other signalling molecules.
Similar content being viewed by others
Abbreviations
- 3-MPA:
-
3-Mercaptopropionic acid
- ALMT:
-
Aluminium (Al3+)-activated malate transporter
- C/Cys:
-
Cysteine
- EC50 :
-
Half-maximal response
- F/Phe:
-
Phenylalanine
- GABA:
-
γ-aminobutyric acid
- GABA-T:
-
GABA transaminase
- GABP:
-
GABA permease
- GAD:
-
Glutamate decarboxylase
- GAT:
-
GABA transporter
- GDH:
-
Glutamate dehydrogenase
- E/Glu:
-
Glutamic acid
- I/Ile:
-
Isoleucine
- SSA:
-
Succinic semialdehyde
- SSADH:
-
Succinic semialdehyde dehydrogenase
- T/Thr:
-
Threonine
- D/Asp:
-
Aspartic acid
- V/Val:
-
Valine
- Y/Tyr:
-
Tyrosine
- Q/Gln:
-
Glutamine
- L/Leu:
-
Leucine
- R/Arg:
-
Arginine
- TMDs:
-
Transmembrane domains
- K/Lys:
-
Lysine
- S/Ser:
-
Serine
- G/Gly:
-
Glycine
References
Steward F, Thompson J, Dent C (1949) γ-Aminobutyric acid, a constituent of the potato tuber. Science 110:439–440
Roberts E, Frankel S (1950) γ-Aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem 187:55–63
Awapara J, Landua AJ, Fuerst R, Seale B (1950) Free γ-aminobutyric acid in brain. J Biol Chem 187:35–39
Elliott K, Jasper HH (1959) Gamma-aminobutyric acid. Physiol Rev 39(2):383–406
Bloom F, Iversen L (1971) Localizing 3H-GABA in nerve terminals of rat cerebral cortex by electron microscopic autoradiography. Nature 229:628–630
Palacios JM, Wamsley JK, Kuhar MJ (1981) High affinity GABA receptors—autoradiographic localization. Brain Res 222(2):285–307
Watanabe M, Fukuda A (2015) Development and regulation of chloride homeostasis in the central nervous system. Front Cell Neurosci 9:14. doi:10.3389/fncel.2015.00371
Cooper P, Selman I (1974) An analysis of the effects of tobacco mosaic virus on growth and the changes in the free amino compounds in young tomato plants. Ann Bot 38:625–638
Ben-Ari Y, Gaiarsa J-L, Tyzio R, Khazipov R (2007) GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev 87(4):1215–1284
Li K, Xu E (2008) The role and the mechanism of γ-aminobutyric acid during central nervous system development. Neurosci Bull 24(3):195–200
Erdö SL, De Vincentis G, Amenta F (1990) Autoradiographic localization of [3 H] muscimol binding sites in rat stomach: evidence for mucosal GABAA receptors. Eur J Pharmacol 175(3):351–354
Barragan A, Weidner JM, Jin Z, Korpi E, Birnir B (2015) GABAergic signalling in the immune system. Acta Physiol 213(4):819–827
Owens DF, Kriegstein AR (2002) Is there more to GABA than synaptic inhibition? Nat Rev Neurosci 3(9):715–727
Shelp BJ, Bown AW, McLean MD (1999) Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 4(11):446–452
Bouché N, Fait A, Bouchez D, Møller SG, Fromm H (2003) Mitochondrial succinic-semialdehyde dehydrogenase of the γ-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc Natl Acad Sci USA 100(11):6843–6848
Bouche N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9(3):110–115. doi:10.1016/j.tplants.2004.01.006
Bown A, Shelp B (1997) The metabolism and functions of γ-aminobutyric acid. Plant Physiol Biochem 115:1–5
Ramesh SA, Tyerman SD, Xu B, Bose J, Kaur S, Conn V, Domingos P, Ullah S, Wege S, Shabala S, Feijo JA, Ryan PR, Gilliham M (2015) GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat Commun. doi:10.1038/ncomms8879
Palanivelu R, Brass L, Edlund AF, Preuss D (2003) Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114(1):47–59
Yue X, Gao XQ, Wang F, Dong Y, Li X, Zhang XS (2014) Transcriptional evidence for inferred pattern of pollen tube-stigma metabolic coupling during pollination. PLoS One 9(9):e107046. doi:10.1371/journal.pone.0107046
Shelp BJ, Mullen RT, Waller JC (2012) Compartmentation of GABA metabolism raises intriguing questions. Trends Plant Sci 17(2):57–59. doi:10.1016/j.tplants.2011.12.006
Kinnersley AM, Turano FJ (2000) Gamma aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci 19(6):479–509. doi:10.1080/07352680091139277
Kinnersley AM (1999) Physiological evidence for GABA receptors in plants. Plant Biol 1999:153
Bouche N, Lacombe B, Fromm H (2003) GABA signaling: a conserved and ubiquitous mechanism. Trends Cell Biol 13(12):607–610
Shelp BJ, Bozzo GG, Trobacher CP, Zarei A, Deyman KL, Brikis CJ (2012) Hypothesis/review: contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci 193–194:130–135. doi:10.1016/j.plantsci.2012.06.001
Bown AW, Shelp BJ (2016) Plant GABA: not just a metabolite. Trend Plant Sci
Gilliham M, Tyerman SD (2015) Linking metabolism to membrane signaling: the GABA—malate connection. Trends Plant Sci
Žárský V (2015) Signal transduction: GABA receptor found in plants. Nat Plants 1:15115
Yin YG, Tominaga T, Iijima Y, Aoki K, Shibata D, Ashihara H, Nishimura S, Ezura H, Matsukura C (2010) Metabolic alterations in organic acids and gamma-aminobutyric acid in developing tomato (Solanum lycopersicum L.) fruits. Plant Cell Physiol 51(8):1300–1314. doi:10.1093/pcp/pcq090
Code RA, Burd GD, Rubel EW (1989) Development of GABA immunoreactivity in brainstem auditory nuclei of the chick: ontogeny of gradients in terminal staining. J Comp Neurol 284(4):504–518
Johnston GA (1996) GABAC receptors: relatively simple transmitter-gated ion channels? Trends Pharmacol Sci 17(9):319–323
Zhang W, Ryan P, Sasaki T, Yamamoto Y, Sullivan W, Tyerman S (2008) Characterisation of the TaALMT1 protein as an Al3+-activated anion channel in transformed tobacco (Nicotiana tabacum L.) cells. Plant Cell Physiol 49:1316–1330
Pineros MA, Cançado GM, Kochian LV (2008) Novel properties of the wheat aluminum tolerance organic acid transporter (TaALMT1) revealed by electrophysiological characterization in Xenopus oocytes: functional and structural implications. Plant Physiol 147(4):2131–2146
Meyer S, Mumm P, Imes D, Endler A, Weder B, Al-Rasheid KAS, Geiger D, Marten I, Martinoia E, Hedrich R (2010) AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J 63(6):1054–1062. doi:10.1111/j.1365-313X.2010.04302.x
Cho MH, Spalding EP (1996) An anion channel in Arabidopsis hypocotyls activated by blue light. Proc Natl Acad Sci USA 93(15):8134–8138
Thomine S, Lelièvre F, Boufflet M, Guern J, Barbier-Brygoo H (1997) Anion-channel blockers interfere with auxin responses in dark-grown Arabidopsis hypocotyls. Plant Physiol 115(2):533–542
Colcombet J, Mathieu Y, Peyronnet R, Agier N, Lelièvre F, Barbier-Brygoo H, Frachisse J-M (2009) R-type anion channel activation is an essential step for ROS-dependent innate immune response in Arabidopsis suspension cells. Funct Plant Biol 36(9):832–843
Barbier-Brygoo H, De Angeli A, Filleur S, Frachisse JM, Gambale F, Thomine S, Wege S (2011) Anion channels/transporters in plants: from molecular bases to regulatory networks. Annu Rev Plant Biol 62:25–51. doi:10.1146/annurev-arplant-042110-103741
Kollist H, Jossier M, Laanemets K, Thomine S (2011) Anion channels in plant cells. FEBS J 278(22):4277–4292
Bormann J (1988) Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci 11(3):112–116
Bowery NG, Doble A, Hill DR, Hudson AL, Shaw JS, Turnbull MJ, Warrington R (1981) Bicuculline-insensitive GABA receptors on peripheral autonomic nerve terminals. Eur J Pharmacol 71(1):53–70
Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa J-L (1997) GABAA, NMDA and AMPA receptors: a developmentally regulatedménage à trois’. Trends Neurosci 20(11):523–529
Ben-Ari Y (2002) Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci 3(9):728–739
Bouche N, Fait A, Zik M, Fromm H (2004) The root-specific glutamate decarboxylase (GAD1) is essential for sustaining GABA levels in Arabidopsis. Plant Mol Biol 55(3):315–325. doi:10.1007/s11103-004-0650-z
Lam H-M, Chiu J, Hsieh M-H, Meisel L, Oliveira IC, Shin M, Coruzzi G (1998) Glutamate-receptor genes in plants. Nature 396(6707):125–126
Lacombe B, Becker D, Hedrich R, DeSalle R, Hollmann M, Kwak JM, Schroeder JI, Le Novère N, Nam HG, Spalding EP (2001) The identity of plant glutamate receptors. Science 292(5521):1486
Turano FJ, Panta GR, Allard MW, van Berkum P (2001) The putative glutamate receptors from plants are related to two superfamilies of animal neurotransmitter receptors via distinct evolutionary mechanisms. Mol Biol Evol 18(7):1417–1420
Dubos C, Huggins D, Grant GH, Knight MR, Campbell MM (2003) A role for glycine in the gating of plant NMDA-like receptors. Plant J 35(6):800–810
Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, Gilliham M, Liu LH, Obermeyer G, Feijo JA (2011) Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 332(6028):434–437. doi:10.1126/science.1201101
Kim SA, Kwak J, Jae S-K, Wang M-H, Nam H (2001) Overexpression of the AtGluR2 gene encoding an Arabidopsis homolog of mammalian glutamate receptors impairs calcium utilization and sensitivity to ionic stress in transgenic plants. Plant Cell Physiol 42(1):74–84
Demidchik V, Essah PA, Tester M (2004) Glutamate activates cation currents in the plasma membrane of Arabidopsis root cells. Planta 219(1):167–175
Dennison KL, Spalding EP (2000) Glutamate-gated calcium fluxes in Arabidopsis. Plant Physiol 124(4):1511–1514
Lancien M, Roberts MR (2006) Regulation of Arabidopsis thaliana 14-3-3 gene expression by gamma-aminobutyric acid. Plant Cell Environ 29(7):1430–1436. doi:10.1111/j.1365-3040.2006.01526.x
Laha KT, Tran PN (2013) Multiple tyrosine residues at the GABA binding pocket influence surface expression and mediate kinetics of the GABAA receptor. J Neurochem 124(2):200–209. doi:10.1111/jnc.12083
Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell 14:S401–S417
Kovermann P, Meyer S, Hortensteiner S, Picco C, Scholz-Starke J, Ravera S, Lee YEM (2007) The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J 52:1169–1180
De Angeli A, Zhang J, Meyer S, Martinoia E (2013) AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat Commun 4:1804
Dreyer I, Gomez-Porras JL, Riaño-Pachón DM, Hedrich R, Geiger D (2013) Molecular evolution of slow and quick anion channels (SLACs and QUACs/ALMTs). Front Plant Sci:97
Hedrich R (2012) Ion channels in plants. Physiol Rev 92(4):1777–1811
Gutermuth T, Lassig R, Portes M-T, Maierhofer T, Romeis T, Borst J-W, Hedrich R, Feijó JA, Konrad KR (2013) Pollen tube growth regulation by free anions depends on the interaction between the anion channel SLAH3 and calcium-dependent protein kinases CPK2 and CPK20. Plant Cell 25(11):4525–4543
De Angeli A, Baetz U, Francisco R, Zhang J, Chaves MM, Regalado A (2013) The vacuolar channel VvALMT9 mediates malate and tartrate accumulation in berries of Vitis vinifera. Planta 238(2):283–291
Shelp BJ, Bozzo GG, Zarei A, Simpson JP, Trobacher CP, Allan WL (2012) Strategies and tools for studying the metabolism and function of γ-aminobutyrate in plants. II. Integrated analysis. Botany 90(9):781–793
Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg L, Schaeffer JM, Arena JP (1994) Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 371(6499):707–711
Hilf RJ, Dutzler R (2009) Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457(7225):115–118
Miller PS, Aricescu AR (2014) Crystal structure of a human GABAA receptor. Nature 512(7514):270–275
Schofield PR, Darlison MG, Fujita N, Burt DR, Stephenson FA, Rodriguez H, Rhee LM, Ramachandran J, Reale V, Glencorse TA (1987) Sequence and functional expression of the GABAA receptor shows a ligand-gated receptor super-family. Nature 328:221–227. doi:10.1038/328221a0
Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Höger H, Adamiker D (1999) Structure and subunit composition of GABAA receptors. Neurochem Int 34(5):379–385
Sieghart W (2006) Structure, pharmacology, and function of GABAA receptor subtypes. Adv Pharmacol 54:231
Smith GB, Olsen RW (1995) Functional domains of GABAA receptors. Trends Pharmacol Sci 16(5):162–168
Cromer BA, Morton CJ, Parker MW (2002) Anxiety over GABAA receptor structure relieved by AChBP. Trends Biochem Sci 27(6):280–287
Sieghart W, Sperk G (2002) Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem 2(8):795–816
Whiting P (1999) The GABAA receptor gene family: new targets for therapeutic intervention. Neurochem Int 34(5):387–390
Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger W, Hoger H, Adamiker D (1999) Structure and subunit composition of GABAA receptors. Neurochem Int 34:379–385
Brickley S, Farrant M, Swanson G, Cull-Candy S (2001) CNQX increases GABA-mediated synaptic transmission in the cerebellum by an AMPA/kainate receptor-independent mechanism. Neuropharmacol 41(6):730–736
Stell BM, Brickley SG, Tang C, Farrant M, Mody I (2003) Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci USA 100(24):14439–14444
Farrant M, Nusser Z (2005) Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci 6(3):215–229
Horenstein J, Wagner DA, Czajkowski C, Akabas MH (2001) Protein mobility and GABA-induced conformational changes in GABAA receptor pore-lining M2 segment. Nat Neurosci 4(5):477–485
Chang Y, Weiss DS (2002) Site-specific fluorescence reveals distinct structural changes with GABA receptor activation and antagonism. Nat Neurosci 5(11):1163–1168
Amin J, Dickerson I, Weiss DS (1994) The agonist binding site of the gamma-aminobutyric acid type A channel is not formed by the extracellular cysteine loop. Mol Pharmacol 45(2):317–323
Sumikawa K, Gehle VM (1992) Assembly of mutant subunits of the nicotinic acetylcholine receptor lacking the conserved disulfide loop structure. J Biol Chem 267(9):6286–6290
Vandenberg RJ, Rajendra S, French CR, Barry PH, Schofield PR (1993) The extracellular disulfide loop motif of the inhibitory glycine receptor does not form the agonist binding site. Mol Pharm 44(1):198–203
Miller SM, Piasecki CC, Peabody MF, Lonstein JS (2010) GABAA receptor antagonism in the ventrocaudal periaqueductal gray increases anxiety in the anxiety-resistant postpartum rat. Pharmacol Biochem Behav 95(4):457–465
Macdonald RL, Olsen RW (1994) GABAA receptor channels. Annu Rev Neurosci 17(1):569–602
Rabow LE, Russek SJ, Farb DH (1995) From ion currents to genomic analysis: recent advances in GABAA receptor research. Synapse 21(3):189–274
Chen ZW, Olsen RW (2007) GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J Neurochem 100(2):279–294
Jacob TC, Moss SJ, Jurd R (2008) GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci 9(5):331–343
Boileau AJ, Evers AR, Davis AF, Czajkowski C (1999) Mapping the agonist binding site of the GABAA receptor: evidence for a β-strand. J Neurosci 19(12):4847–4854
Motoda H, Sasaki T, Kano Y, Ryan PR, Delhaize E, Matsumoto H, Yamamoto Y (2007) The membrane topology of ALMT1, an aluminum-activated malate transport protein in wheat (Triticum aestivum). Plant Signal Behav 2(6):467–472
Mumm P, Imes D, Martinoia E, Al-Rasheid KA, Geiger D, Marten I, Hedrich R (2013) C-terminus-mediated voltage gating of Arabidopsis guard cell anion channel QUAC1. Mol Plant 6(5):1550–1563
Zhang J, Baetz U, Krügel U, Martinoia E, De Angeli A (2013) Identification of a probable pore-forming domain in the multimeric vacuolar anion channel AtALMT9. Plant Physiol 163(2):830–843
Smith GB, Olsen RW (1994) Identification of a [3H] muscimol photoaffinity substrate in the bovine gamma-aminobutyric acid A receptor alpha subunit. J Biol Chem 269(32):20380–20387
Sigel E, Baur R, Kellenberger S, Malherbe P (1992) Point mutations affecting antagonist affinity and agonist dependent gating of GABAA receptor channels. EMBO J 11(6):2017
Szczot M, Kisiel M, Czyzewska MM, Mozrzymas JW (2014) α1F64 Residue at GABAA receptor binding site is involved in gating by influencing the receptor flipping transitions. J Neurosci 34(9):3193–3209
de Ruijter NC, Malhó R (2000) F-box proteins in Arabidopsis. Cell 5(11):1360–1385. doi:10.1016/S1360-1385(00)01769-6
Lamberti G, Gügel IL, Meurer J, Soll J, Schwenkert S (2011) The cytosolic kinases STY8, STY17, and STY46 are involved in chloroplast differentiation in Arabidopsis. Plant Physiol 157(1):70–85
Marks DS, Colwell LJ, Sheridan R, Hopf TA, Pagnani A, Zecchina R, Sander C (2011) Protein 3D structure computed from evolutionary sequence variation. PLoS One 6(12):e28766
Hopf TA, Colwell LJ, Sheridan R, Rost B, Sander C, Marks DS (2012) Three-dimensional structures of membrane proteins from genomic sequencing. Cell 149(7):1607–1621
Marks DS, Hopf TA, Sander C (2012) Protein structure prediction from sequence variation. Nat Biotechnol 30(11):1072–1080
Meier J, Vannier C, Serge A, Triller A, Choquet D (2001) Fast and reversible trapping of surface glycine receptors by gephyrin. Nat Neurosci 4(3):253–260
Borgdorff AJ, Choquet D (2002) Regulation of AMPA receptor lateral movements. Nature 417(6889):649–653
Perez-Velazquez JL, Angelides KJ (1993) Assembly of GABAA receptor subunits determines sorting and localization in polarized cells. Nature 361:457–460. doi:10.1038/361457a0
Barnes EM (2000) Intracellular trafficking of GABAA receptors. Life Sci 66(12):1063–1070
Kittler JT, Moss SJ (2003) Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Chem Biol 13(3):341–347
Jalilian Tehrani MH, Barnes EM (1993) Identification of GABAA/benzodiazepine receptors on clathrin-coated vesicles from rat rrain. J Neurochem 60(5):1755–1761
Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ (2000) Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci 20(21):7972–7977
Herring D, Huang R, Singh M, Robinson LC, Dillon GH, Leidenheimer NJ (2003) Constitutive GABAA receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin-binding motif within the β2 subunit of the receptor. J Biol Chem 278(26):24046–24052
Tehrani MHJ, Barnes EM (1991) Agonist-dependent internalization of γ-aminobutyric acid A/benzodiazepine receptors in chick cortical neurons. J Neurochem 57(4):1307–1312
Calkin PA, Baumgartner BJ, Barnes EM (1994) Agonist administration in ovo down-regulates cerebellar GABAA receptors in the chick embryo. Mol Brain Res 26(1):18–25
Johnston GA (1996) GABAA receptor pharmacology. Pharmacol Ther 69(3):173–198
Johnston GA, Hanrahan JR, Chebib M, Duke RK, Mewett KN (2006) Modulation of ionotropic GABA receptors by natural products of plant origin. Adv Pharmacol 54:285
Grant SM, Heel RC (1991) Vigabatrin. Drugs 41(6):889–926
Katoh J, Taniguchi H, Ogura M, Kasuga M, Okada Y (1995) A convulsant, 3-mercaptopropionic acid, decreases the level of GABA and GAD in rat pancreatic islets and brain. Experientia 51(3):217–219
Barker JL, Mathers DA (1981) GABA analogues activate channels of different duration on cultured mouse spinal neurons. Science 212(4492):358–361
Jackson MB, Lecar H, Mathers DA, Barker JL (1982) Single channel currents activated by gamma-aminobutyric acid, muscimol, and (−)-pentobarbital in cultured mouse spinal neurons. J Neurosci 2(7):889–894
Khawaled R, Bruening-Wright A, Adelman JP, Maylie J (1999) Bicuculline block of small-conductance calcium-activated potassium channels. Pflügers Archiv 438(3):314–321
Barker J, McBurney R, Mathers D (1983) Convulsant-induced depression of amino acid responses in cultured mouse spinal neurones studied under voltage clamp. Br J Pharmacol 80(4):619–629
Crawley J, Goodwin FK (1980) Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav 13(2):167–170
Hoffman E, Warren E (1993) Flumazenil: a benzodiazepine antagonist. Clin Pharm 12(9):641–656 (quiz 699–701)
Bowden K, Drysdale A (1965) A novel constituent of Amanitamuscaria. Tetrahedron Lett 6(12):727–728
Nayak P, Chatterjee A (2001) Effects of aluminium exposure on brain glutamate and GABA systems: an experimental study in rats. Food Chem Toxicol 39(12):1285–1289
Flaten TP, Alfrey AC, Birchall JD, Savory J, Yokel RA (1996) Status and future concerns of clinical and environmental aluminum toxicology. J Toxicol Environ Health A 48(6):527–542
Organisation WH (1997) Environment health criteria 194. Aluminium. WHO, Geneva
Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Biol 46(1):237–260
Delhaize E, Ryan PR (1995) Aluminum toxicity and tolerance in plants. Plant Physiol 107(2):315
Kochian LV, Pineros MA, Hoekenga OA (2005) The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Root physiology: from gene to function. Springer, New York, pp 175–195
Alfrey AC, LeGendre GR, Kaehny WD (1976) The dialysis encephalopathy syndrome: possible aluminum intoxication. N Engl J Med 294(4):184–188
Berlyne G (1989) Dialysis in the third world. Nephron 53(1):1
Bolla KI, Briefel G, Spector D, Schwartz BS, Wieler L, Herron J, Gimenez L (1992) Neurocognitive effects of aluminum. Arch Neurol 49(10):1021–1026
Trombley PQ (1998) Selective modulation of GABAA receptors by aluminum. J Neurophysiol 80(2):755–761
Horst W, Wagner A, Marschner H (1983) Effect of aluminium on root growth, cell-division rate and mineral element contents in roots of Vigna unguiculata genotypes. Zeitschrift für Pflanzenphysiologie 109(2):95–103
Ryan P, Delhaize E, Jones D (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Biol 52(1):527–560
Čiamporová M (2002) Morphological and structural responses of plant roots to aluminium at organ, tissue, and cellular levels. Biol Plantarum 45(2):161–171
Platt B, Büsselberg D (1994) Actions of aluminum on voltage-activated calcium channel currents. Cell Mol Neurobiol 14(6):819–829
Platt B, Haas H, Büsselberg D (1994) Aluminium reduces glutamate-activated currents of rat hippocampal neurones. NeuroReport 5(17):2329–2332
Candy J, Klinowski J, Perry R, Perry E, Fairbairn A, Oakley A, Carpenter T, Atack J, Blessed G, Edwardson J (1986) Aluminosilicates and senile plaque formation in Alzheimer’s disease. Lancet 327(8477):354–356
Perl DP, Gajdusek DC, Garruto RM, Yanagihara RT, Gibbs CJ (1982) Intraneuronal aluminum accumulation in amyotrophic lateral sclerosis and Parkinsonism-dementia of Guam. Science 217(4564):1053–1055
El-Rahman SSA (2003) Neuropathology of aluminum toxicity in rats (glutamate and GABA impairment). Pharmacol Res 47(3):189–194
Ma JF, Ryan PR, Delhaize E (2001) Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6(6):273–278
Ryan PR, Tyerman SD, Sasaki T, Furuichi T, Yamamoto Y, Zhang W, Delhaize E (2011) The identification of aluminium-resistance genes provides opportunities for enhancing crop production on acid soils. J Exp Bot 62(1):9–20
Michaeli S, Fait A, Lagor K, Nunes-Nesi A, Grillich N, Yellin A, Bar D, Khan M, Fernie AR, Turano FJ (2011) A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J 67(3):485–498
Meyer A, Eskandari S, Grallath S, Rentsch D (2006) AtGAT1, a high affinity transporter for γ-aminobutyric acid in Arabidopsis thaliana. J Biol Chem 281(11):7197–7204
Cordeiro J, Silva V, Oliveira C, Goncalves P (2003) Aluminium-induced impairment of Ca2+ modulatory action on GABA transport in brain cortex nerve terminals. J Inorg Biochem 97(1):132–142
Ohta T (1989) Role of gene duplication in evolution. Genome 31(1):304–310
Otto SP, Whitton J (2000) Polyploid incidence and evolution. Annu Rev Genet 34(1):401–437
Taylor JS, Raes J (2004) Duplication and divergence: the evolution of new genes and old ideas. Annu Rev Genet 38:615–643
Shimeld SM, Holland PW (2000) Vertebrate innovations. Proc Natl Acad Sci USA 97(9):4449–4452
Ortells MO, Lunt GG (1995) Evolutionary history of the ligand-gated ion-channel superfamily of receptors. Trends Neurosci 18(3):121–127
Russek SJ (1999) Evolution of GABA A receptor diversity in the human genome. Gene 227(2):213–222
Darlison MG, Pahal I, Thode C (2005) Consequences of the evolution of the GABAA receptor gene family. Cell Mol Neurobiol 25(3–4):607–624
Martyniuk CJ, Aris-Brosou S, Drouin G, Cahn J, Trudeau VL (2007) Early evolution of ionotropic GABA receptors and selective regimes acting on the mammalian-specific theta and epsilon subunits. PLoS One 2(9):e894
Wendel JF (2000) Genome evolution in polyploids. Plant molecular evolution. Springer, New York, pp 225–249
Ku H-M, Vision T, Liu J, Tanksley SD (2000) Comparing sequenced segments of the tomato and Arabidopsis genomes: large-scale duplication followed by selective gene loss creates a network of synteny. Proc Natl Acad Sci USA 97(16):9121–9126
Vision TJ, Brown DG, Tanksley SD (2000) The origins of genomic duplications in Arabidopsis. Science 290(5499):2114–2117
Bowers JE, Chapman BA, Rong J, Paterson AH (2003) Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature 422(6930):433–438
Soltis DE, Soltis PS, Endress PK, Chase MW (2005) Phylogeny and evolution of angiosperms. Sinauer Associates Incorporated
Blanc G, Agarkova I, Grimwood J, Kuo A, Brueggeman A, Dunigan DD, Gurnon J, Ladunga I, Lindquist E, Lucas S (2012) The genome of the polar eukaryotic microalga Coccomyxa subellipsoidea reveals traits of cold adaptation. Genome Biol 13(5):R39
Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, Geer RC, He J, Gwadz M, Hurwitz DI (2014) CDD: NCBI’s conserved domain database. Nucleic Acids Res:gku1221
Baum G, Lev-Yadun S, Fridmann Y, Arazi T, Katsnelson H, Zik M, Fromm H (1996) Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J 15(12):2988–2996
Bown AW, Zhang G (2000) Mechanical stimulation, 4-aminobutyric acid (GABA) synthesis, and growth inhibition in soybean hypocotyl tissue. Can J Bot 78(1):119–123
Kathiresan A, Tung P, Chinnappa CC, Reid DM (1997) gamma-aminobutyric acid stimulates ethylene biosynthesis in sunflower. Plant Physiol 115(1):129–135. doi:10.1104/pp.115.1.129
Renault H, Roussel V, El Amrani A, Arzel M, Renault D, Bouchereau A, Deleu C (2010) The Arabidopsis pop2-1 mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant Biol 10(1):20
Renault H, El Amrani A, Palanivelu R, Updegraff EP, Yu A, Renou JP, Preuss D, Bouchereau A, Deleu C (2011) GABA accumulation causes cell elongation defects and a decrease in expression of genes encoding secreted and cell wall-related proteins in Arabidopsis thaliana. Plant Cell Physiol 52(5):894–908. doi:10.1093/pcp/pcr041
Michaeli S, Fromm H (2015) Closing the loop on the GABA shunt in plants: are GABA metabolism and signaling entwined? Front Plant Sci 6
Beuve N, Rispail N, Laine P, Cliquet JB, Ourry A, Le Deunff E (2004) Putative role of γ-aminobutyric acid (GABA) as a long-distance signal in up-regulation of nitrate uptake in Brassica napus L. Plant Cell Environ 27(8):1035–1046
Barbosa JM, Singh NK, Cherry JH, Locy RD (2000) GABA increases the rate of nitrate uptake and utilization in Arabidopsis roots. Plant Biol 2000:133
Barbosa JM, Singh NK, Cherry JH, Locy RD (2010) Nitrate uptake and utilization is modulated by exogenous gamma-aminobutyric acid in Arabidopsis thaliana seedlings. Plant Physiol Biochem 48(6):443–450. doi:10.1016/j.plaphy.2010.01.020
Jin H, Dilworth M, Glenn A (1990) 4-Aminobutyrate is not available to bacteroids of cowpea Rhizobium MNF2030 in snake bean nodules. Arch Microbiol 153(5):455–462
Miller R, McRae D, Joy K (1991) Glutamate and gamma-aminobutyrate metabolism in isolated Rhizobium meliloti bacteroids. Mol Plant-Microbe Interact 4:37–45
Sulieman S (2011) Does GABA increase the efficiency of symbiotic N2 fixation in legumes? Plant Signal Behav 6(1):32–36
Diaz C, Lemaître T, Christ A, Azzopardi M, Kato Y, Sato F, Morot-Gaudry J-F, Le Dily F, Masclaux-Daubresse C (2008) Nitrogen recycling and remobilization are differentially controlled by leaf senescence and development stage in Arabidopsis under low nitrogen nutrition. Plant Physiol 147(3):1437–1449
Allan WL, Shelp BJ (2006) A potential role for gamma-hydroxybuty rate production in redox homeostasis. Plant Biol 2006:219
Ling Y, Chen T, Jing Y, Fan L, Wan Y, Lin J (2013) γ-Aminobutyric acid (GABA) homeostasis regulates pollen germination and polarized growth in Picea wilsonii. Planta 238(5):831–843
Frietsch S, Wang Y-F, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, Harper JF (2007) A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci USA 104(36):14531–14536
Schiøtt M, Romanowsky SM, Bækgaard L, Jakobsen MK, Palmgren MG, Harper JF (2004) A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc Natl Acad Sci USA 101(25):9502–9507
Song L-F, Zou J-J, Zhang W-Z, Wu W-H, Wang Y (2009) Ion transporters involved in pollen germination and pollen tube tip-growth. Plant Signal Behav 4(12):1193–1195
Saikusa T, Horino T, Mori Y (1994) Distribution of free amino acids in the rice kernel and kernel fractions and the effect of water soaking on the distribution. J Agric Food Chem 42(5):1122–1125
Dluzniewska P, Gessler A, Kopriva S, Strnad M, Novak O, Dietrich H, Rennenberg H (2006) Exogenous supply of glutamine and active cytokinin to the roots reduces NO3 − uptake rates in poplar. Plant Cell Environ 29(7):1284–1297
Mazzucotelli E, Tartari A, Cattivelli L, Forlani G (2006) Metabolism of gamma-aminobutyric acid during cold acclimation and freezing and its relationship to frost tolerance in barley and wheat. J Exp Bot 57(14):3755–3766. doi:10.1093/jxb/erl141
Vannini C, Iriti M, Bracale M, Locatelli F, Faoro F, Croce P, Pirona R, Di Maro A, Coraggio I, Genga A (2006) The ectopic expression of the rice Osmyb4 gene in Arabidopsis increases tolerance to abiotic, environmental and biotic stresses. Physiol Mol Plant Pathol 69(1):26–42
Xing SG, Jun YB, Hau ZW, Liang LY (2007) Higher accumulation of γ-aminobutyric acid induced by salt stress through stimulating the activity of diamine oxidases in Glycine max (L.) Merr. roots. Plant Physiol Biochem 45(8):560–566
Bor M, Seckin B, Ozgur R, Yilmaz O, Ozdemir F, Turkan I (2009) Comparative effects of drought, salt, heavy metal and heat stresses on gamma-aminobutryric acid levels of sesame (Sesamum indicum L.). Acta Physiol Plant 31(3):655–659. doi:10.1007/s11738-008-0255-2
Patterson JH, Newbigin E, Tester M, Bacic A, Roessner U (2009) Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J Exp Bot:erp243
Al-Quraan NA, Locy RD, Singh NK (2011) Implications of paraquat and hydrogen peroxide-induced oxidative stress treatments on the GABA shunt pathway in Arabidopsis thaliana calmodulin mutants. Plant Biotechnol Rep 5(3):225–234
Akçay N, Bor M, Karabudak T, Özdemir F, Türkan I (2012) Contribution of gamma amino butyric acid (GABA) to salt stress responses of Nicotiana sylvestris CMSII mutant and wild type plants. J Plant Physiol 169(5):452–458
Guo Y, Yang R, Chen H, Song Y, Gu Z (2012) Accumulation of γ-aminobutyric acid in germinated soybean (Glycine max L.) in relation to glutamate decarboxylase and diamine oxidase activity induced by additives under hypoxia. Eur Food Res Technol 234(4):679–687
Vergara R, Parada F, Rubio S, Perez FJ (2012) Hypoxia induces H2O2 production and activates antioxidant defence system in grapevine buds through mediation of H2O2 and ethylene. J Exp Bot 63(11):4123–4131. doi:10.1093/jxb/ers094
Nayyar H, Kaur R, Kaur S, Singh R (2014) γ-Aminobutyric acid (GABA) imparts partial protection from heat stress injury to rice seedlings by improving leaf turgor and upregulating osmoprotectants and antioxidants. J Plant Growth Reg 33(2):408–419
Mekonnen DW, Flügge U-I, Ludewig F (2016) Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci 245:25–34
Fougere F, Le Rudulier D, Streeter JG (1991) Effects of salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.). Plant Physiol 96(4):1228–1236
Bolarín MC, Santa-Cruz A, Cayuela E, Perez-Alfocea F (1995) Short-term solute changes in leaves and roots of cultivated and wild tomato seedlings under salinity. J Plant Physiol 147(3):463–468
Widodo, Patterson JH, Newbigin E, Tester M, Bacic A, Roessner U (2009) Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J Exp Bot 60(14):4089–4103. doi:10.1093/jxb/erp243
Zhang J, Zhang Y, Du Y, Chen S, Tang H (2011) Dynamic metabonomic responses of tobacco (Nicotiana tabacum) plants to salt stress. J Proteome Res 10(4):1904–1914. doi:10.1021/pr101140n
Renault H, El Amrani A, Berger A, Mouille G, Soubigou-Taconnat L, Bouchereau A, Deleu C (2013) gamma-Aminobutyric acid transaminase deficiency impairs central carbon metabolism and leads to cell wall defects during salt stress in Arabidopsis roots. Plant Cell Environ 36(5):1009–1018. doi:10.1111/pce.12033
Baetz U, Eisenach C, Tohge T, Martinoia E, De Angeli A (2016) Vacuolar chloride fluxes impact ion content and distribution during early salinity stress. Plant Physiol:00183.02016
Raggi V (1994) Changes in free amino acids and osmotic adjustment in leaves of water-stressed bean. Physiol Plant 91(3):427–434
Serraj R, Shelp BJ, Sinclair TR (1998) Accumulation of gamma-aminobutyric acid in nodulated soybean in response to drought stress. Physiol Plant 102(1):79–86. doi:10.1034/j.1399-3054.1998.1020111.x
Thompson JF, Stewart CR, Morris CJ (1966) Changes in amino acid content of excised leaves during incubation I. The effect of water content of leaves and atmospheric oxygen level. Plant Physiol 41(10):1578–1584
Scholz SS, Reichelt M, Mekonnen DW, Ludewig F, Mithöfer A (2015) Insect herbivory-elicited GABA accumulation in plants is a wound-induced, direct, systemic, and jasmonate-independent defense response. Front Plant Sci 6:1128. doi:10.3389/fpls.2015.01128
Salvatierra A, Pimentel P, Almada R, Hinrichsen P (2016) Exogenous GABA application transiently improves the tolerance to root hypoxia on a sensitive genotype of Prunus rootstock. Environ Exp Bot 125:52–66
Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ (1993) Aluminum tolerance in wheat (Triticum aestivum L.)(I. Uptake and distribution of aluminum in root apices). Plant Physiol 103(3):685–693
Delhaize E, Ryan PR, Randall PJ (1993) Aluminum tolerance in wheat (Triticum aestivum L.) (II. Aluminum-stimulated excretion of malic acid from root apices). Plant Physiol 103(3):695–702
Sasaki TYY, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminium-activated malate transporter. Plant J 37:645–653
Warren C (2015) Wheat roots efflux a diverse array of organic N compounds and are highly proficient at their recapture. Plant Soil. doi:10.1007/s11104-015-2612-4
Badri DV, De-la-Peña C, Lei Z, Manter DK, Chaparro JM, Guimarães RL, Sumner LW, Vivanco JM (2012) Root secreted metabolites and proteins are involved in the early events of plant-plant recognition prior to competition. PLoS One 7(10):e46640
Fourcroy P, Sisó-Terraza P, Sudre D, Savirón M, Reyt G, Gaymard F, Abadía A, Abadia J, Álvarez-Fernández A, Briat JF (2014) Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytol 201(1):155–167
Solomon PS, Oliver RP (2001) The nitrogen content of the tomato leaf apoplast increases during infection by Cladosporium fulvum. Planta 213(2):241–249
McLean MD, Yevtushenko DP, Deschene A, Van Cauwenberghe OR, Makhmoudova A, Potter JW, Bown AW, Shelp BJ (2003) Overexpression of glutamate decarboxylase in transgenic tobacco plants confers resistance to the northern root-knot nematode. Mol Breed 11(4):277–285
Bown AW, MacGregor KB, Shelp BJ (2006) Gamma-aminobutyrate: defense against invertebrate pests? Trends Plant Sci 11(9):424–427
Takahashi H, Matsumura H, Kawai-Yamada M, Uchimiya H (2008) The cell death factor, cell wall elicitor of rice blast fungus (Magnaporthe grisea) causes metabolic alterations including GABA shunt in rice cultured cells. Plant Signal Behav 3(11):945–953
Park DH, Mirabella R, Bronstein PA, Preston GM, Haring MA, Lim CK, Collmer A, Schuurink RC (2010) Mutations in gamma-aminobutyric acid (GABA) transaminase genes in plants or Pseudomonas syringae reduce bacterial virulence. Plant J 64(2):318–330. doi:10.1111/j.1365-313X.2010.04327.x
Bown AW, Hall DE, MacGregor KB (2002) Insect footsteps on leaves stimulate the accumulation of 4-aminobutyrate and can be visualized through increased chlorophyll fluorescence and superoxide production. Plant Physiol 129(4):1430–1434
Irving S, Osborne M, Wilson R (1976) Virtual absence of L-glutamate from the haemoplasm of arthropod blood. Nature 263:431–433
Irving S, Wilson R, Osborne M (1979) Studies on l-glutamate in insect haemolymph. Physiol Entomol 4:231–240
Sattelle DB (1990) GABA receptors of insects. Adv Insect Physiol 22:1–113
von Keyserlingk HC, Willis RJ (1992) The GABA activated Cl-channel in insects as target for insecticide action: a physiological study. Neurotox’91. Springer, New York, pp 79–104
Casida JE (1993) Insecticide action at the GABA-gated chloride channel: recognition, progress, and prospects. Arch Insect Biochem Physiol 22(1–2):13–23
Seifi HS, Curvers K, De Vleesschauwer D, Delaere I, Aziz A, Hofte M (2013) Concurrent overactivation of the cytosolic glutamine synthetase and the GABA shunt in the ABA-deficient sitiens mutant of tomato leads to resistance against Botrytis cinerea. New Phytol 199(2):490–504. doi:10.1111/nph.12283
Chevrot R, Rosen R, Haudecoeur E, Cirou A, Shelp BJ, Ron E, Faure D (2006) GABA controls the level of quorum-sensing signal in Agrobacterium tumefaciens. Proc Natl Acad Sci USA 103(19):7460–7464. doi:10.1073/pnas.0600313103
Yuan ZC, Haudecoeur E, Faure D, Kerr KF, Nester EW (2008) Comparative transcriptome analysis of Agrobacterium tumefaciens in response to plant signal salicylic acid, indole-3-acetic acid and gamma-amino butyric acid reveals signalling cross-talk and Agrobacterium-plant co-evolution. Cell Microbiol 10(11):2339–2354. doi:10.1111/j.1462-5822.2008.01215.x
Planamente S, Mondy S, Hommais F, Vigouroux A, Morera S, Faure D (2012) Structural basis for selective GABA binding in bacterial pathogens. Mol Microbiol 86(5):1085–1099. doi:10.1111/mmi.12043
Planamente S, Vigouroux A, Mondy S, Nicaise M, Faure D, Moréra S (2010) A conserved mechanism of GABA binding and antagonism is revealed by structure-function analysis of the periplasmic binding protein Atu2422 in Agrobacterium tumefaciens. J Biol Chem 285(39):30294–30303
Busov V, Meilan R, Pearce DW, Rood SB, Ma CP, Tschaplinski TJ, Strauss SH (2006) Transgenic modification of gai or rgl1 causes dwarfing and alters gibberellins, root growth, and metabolite profiles in Populus. Planta 224(2):288–299. doi:10.1007/s00425-005-0213-9
Urano K, Maruyama K, Ogata Y, Morishita Y, Takeda M, Sakurai N, Suzuki H, Saito K, Shibata D, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2009) Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J 57(6):1065–1078. doi:10.1111/j.1365-313X.2008.03748.x
Merewitz EB, Du H, Yu W, Liu Y, Gianfagna T, Huang B (2012) Elevated cytokinin content in ipt transgenic creeping bentgrass promotes drought tolerance through regulating metabolite accumulation. J Exp Bot 63(3):1315–1328. doi:10.1093/jxb/err372
Jespersen D, Yu JJ, Huang BR (2015) Metabolite responses to exogenous application of nitrogen, cytokinin, and ethylene inhibitors in relation to heat-induced senescence in Creeping Bentgrass. PLoS One. doi:10.1371/journal.pone.0123744
Sweetlove LJ, Heazlewood JL, Herald V, Holtzapffel R, Day DA, Leaver CJ, Millar AH (2002) The impact of oxidative stress on Arabidopsis mitochondria. Plant J 32(6):891–904. doi:10.1046/j.1365-313X.2002.01474.x
Luo F, Wang Q, Yin C, Ge Y, Hu F, Huang B, Zhou H, Bao G, Wang B, Lu R, Li Z (2015) Differential metabolic responses of Beauveria bassiana cultured in pupae extracts, root exudates and its interactions with insect and plant. J Invertebr Pathol 130:154–164. doi:10.1016/j.jip.2015.01.003
Janzen DJ, Allen LJ, MacGregor KB, Bown AW (2001) Cytosolic acidification and gamma-aminobutyric acid synthesis during the oxidative burst in isolated Asparagus sprengeri mesophyll cells. Can J Bot 79(4):438–443
Kathiresan A, Miranda J, Chinnappa CC, Reid DM (1998) gamma-aminobutyric acid promotes stem elongation in Stellaria longipes: the role of ethylene. Plant Growth Regul 26(2):131–137. doi:10.1023/a:1006107815064
Shi SQ, Shi Z, Jiang ZP, Qi LW, Sun XM, Li CX, Liu JF, Xiao WF, Zhang SG (2010) Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: regulatory roles for H2O2 and ethylene production. Plant Cell Environ 33(2):149–162. doi:10.1111/j.1365-3040.2009.02065.x
Tian Q, Zhang X, Ramesh S, Gilliham M, Tyerman S, Zhang W (2014) Ethylene negatively regulates aluminium-induced malate efflux from wheat roots and tobacco cells transformed with TaALMT1. J Exp Bot 65(9):2415–2426. doi:10.1093/jxb/eru123
Luchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisci acid biosynthesis in Arabidopsis. Plant J 27:325–333
Schwartz SH, LeonKloosterziel KM, Koornneef M, Zeevaart JAD (1997) Biochemical characterization of the aba2 and aba3 mutants in Arabidopsis thaliana. Plant Physiol 114(1):161–166. doi:10.1104/pp.114.1.161
Cao S, Cai Y, Yang Z, Zheng Y (2012) MeJA induces chilling tolerance in loquat fruit by regulating proline and γ-aminobutyric acid contents. Food Chem Toxicol 133(4):1466–1470
Scholz SS, Vadassery J, Heyer M, Reichelt M, Bender KW, Snedden WA, Boland W, Mithöfer A (2014) Mutation of the Arabidopsis calmodulin-like protein CML37 deregulates the jasmonate pathway and enhances susceptibility to herbivory. Mol Plant 7(12):1712–1726
Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R (2009) (+)-7-iso-Jasmonoyl-l-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 5(5):344–350. doi:10.1038/nchembio.161
Smith FA, Raven JA (1979) Intracellular pH and its regulation. Annu Rev Plant Physiol 30(1):289–311
Crawford LA, Bown AW, Breitkreuz KE, Guinel FC (1994) The synthesis of γ-aminobutyric acid in response to treatments reducing cytosolic pH. Plant Physiol 104(3):865–871
Carroll AD, Fox GG, Laurie S, Phillips R, Ratcliffe RG, Stewart GR (1994) Ammonium assimilation and the role of γ-aminobutyric acid in pH homeostasis in carrot cell suspensions. Plant Physiol 106(2):513–520
Kader MA, Lindberg S (2010) Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signal Behav 5(3):233–238
Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu D-T, Bligny R, Maurel C (2003) Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425(6956):393–397
Meyer S, Scholz-Starke J, De Angeli A, Kovermann P, Burla B, Gambale F, Martinoia E (2011) Malate transport by the vacuolar AtALMT6 channel in guard cells is subject to multiple regulation. Plant J 67(2):247–257
Snowden CJ, Thomas B, Baxter CJ, Smith JAC, Sweetlove LJ (2015) A tonoplast Glu/Asp/GABA exchanger that affects tomato fruit amino acid composition. Plant J 81(5):651–660
Dinant S, Suárez-López P (2012) Multitude of long-distance signal molecules acting via phloem. In: Biocommunication of Plants. Springer, New York, pp 89–121
Notaguchi M, Okamoto S (2015) Dynamics of long-distance signaling via plant vascular tissues. Front Plant Sci 6:161
Frak E, Millard P, Le Roux X, Guillaumie S, Wendler R (2002) Coupling sap flow velocity and amino acid concentrations as an alternative method to (15)N labeling for quantifying nitrogen remobilization by walnut trees. Plant Physiol 130(2):1043–1053. doi:10.1104/pp.002139
Queiroz HM, Sodek L, Haddad CRB (2012) Effect of salt on the growth and metabolism of Glycine max. Braz Arch Biol Techn 55(6):809–817
Sulieman S, Schulze J (2010) Phloem-derived gamma-aminobutyric acid (GABA) is involved in upregulating nodule N2 fixation efficiency in the model legume Medicago truncatula. Plant, Cell Environ 33(12):2162–2172. doi:10.1111/j.1365-3040.2010.02214.x
Shelp BJ (2012) Does long-distance GABA signaling via the phloem really occur? Botany 90(10):897–900
Masharina A, Reymond L, Maurel D, Umezawa K, Johnsson K (2012) A fluorescent sensor for GABA and synthetic GABAB receptor ligands. J Am Chem Soc 134(46):19026–19034
Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ (1991) Two genes encode distinct glutamate decarboxylases. Neuron 7(1):91–100
Fon EA, Edwards RH (2001) Molecular mechanisms of neurotransmitter release. Muscle Nerve 24(5):581–601
Cherubini E, Conti F (2001) Generating diversity at GABAergic synapses. Trends Neurosci 24(3):155–162
Roberts E (1988) The establishment of GABA as a neurotransmitter. GABA and benzodiazepine receptors. CRC Press, Boca Raton
Corey JL, Guastella J, Davidson N, Lester HA (1994) GABA uptake and release by a mammalian cell line stably expressing a cloned rat brain GABA transporter. Mol Membr Biol 11(1):23–30
Fenalti G, Law RH, Buckle AM, Langendorf C, Tuck K, Rosado CJ, Faux NG, Mahmood K, Hampe CS, Banga JP (2007) GABA production by glutamic acid decarboxylase is regulated by a dynamic catalytic loop. Nat Struct Mol Biol 14(4):280–286
Akama K, Takaiwa F (2007) C-terminal extension of rice glutamate decarboxylase (OsGAD2) functions as an autoinhibitory domain and overexpression of a truncated mutant results in the accumulation of extremely high levels of GABA in plant cells. J Exp Bot 58(10):2699–2707. doi:10.1093/jxb/erm120
Yu GH, Zou J, Feng J, Peng XB, Wu JY, Wu YL, Palanivelu R, Sun MX (2014) Exogenous gamma-aminobutyric acid (GABA) affects pollen tube growth via modulating putative Ca2+-permeable membrane channels and is coupled to negative regulation on glutamate decarboxylase. J Exp Bot 65(12):3235–3248. doi:10.1093/jxb/eru171
Espinoza C, Degenkolbe T, Caldana C, Zuther E, Leisse A, Willmitzer L, Hincha DK, Hannah MA (2010) Interaction with diurnal and circadian regulation results in dynamic metabolic and transcriptional changes during cold acclimation in Arabidopsis. PLoS One 5(11):e14101
Fait A, Fromm H, Walter D, Galili G, Fernie AR (2008) Highway or byway: the metabolic role of the GABA shunt in plants. Trends Plant Sci 13(1):14–19. doi:10.1016/j.tplants.2007.10.005
Miyashita Y, Good AG (2008) Contribution of the GABA shunt to hypoxia-induced alanine accumulation in roots of Arabidopsis thaliana. Plant Cell Physiol 49(1):92–102. doi:10.1093/pcp/pcm171
Fait A, Yellin A, Fromm H (2005) GABA shunt deficiencies and accumulation of reactive oxygen intermediates: insight from Arabidopsis mutants. FEBS Lett 579(2):415–420. doi:10.1016/j.febslet.2004.12.004
Brozoski TJ, Spires TJD, Bauer CA (2007) Vigabatrin, a GABA transaminase inhibitor, reversibly eliminates tinnitus in an animal model. J Assoc Res Otolaryngol 8(1):105–118
Ludewig F, Hüser A, Fromm H, Beauclair L, Bouché N (2008) Mutants of GABA transaminase (POP2) suppress the severe phenotype of succinic semialdehyde dehydrogenase (ssadh) mutants in Arabidopsis. PLoS ONE 3(10):e3383
Toyokura K, Watanabe K, Oiwaka A, Kusano M, Tameshige T, Tatematsu K, Matsumoto N, Tsugeki R, Saito K, Okada K (2011) Succinic semialdehyde dehydrogenase is involved in the robust patterning of Arabidopsis leaves along the adaxial-abaxial axis. Plant Cell Physiol 52(8):1340–1353. doi:10.1093/pcp/pcr079
Bao H, Chen XY, Lv SL, Jiang P, Feng JJ, Fan PX, Nie LL, Li YX (2015) Virus-induced gene silencing reveals control of reactive oxygen species accumulation and salt tolerance in tomato by gamma-aminobutyric acid metabolic pathway. Plant Cell Environ 38(3):600–613. doi:10.1111/pce.12419
Seher Y, Filiz O, Melike B (2013) Gamma-amino butyric acid, glutamate dehydrogenase and glutamate decarboxylase levels in phylogenetically divergent plants. Plant Syst Evol 299(2):403–412. doi:10.1007/s00606-012-0730-5
Batushansky A, Kirma M, Grillich N, Pham PA, Rentsch D, Galili G, Fernie AR, Fait A (2015) The transporter GAT1 plays an important role in GABA-mediated carbon–nitrogen interactions in Arabidopsis. Front Plant Sci 6:785. doi:10.3389/fpls.2015.00785
Batushansky A, Kirma M, Grillich N, Toubiana D, Pham PA, Balbo I, Fromm H, Galili G, Fernie AR, Fait A (2014) Combined transcriptomics and metabolomics of Arabidopsis thaliana seedlings exposed to exogenous GABA suggest its role in plants is predominantly metabolic. Mol Plant 7(6):1065–1068. doi:10.1093/mp/ssu017
Allan WL, Simpson JP, Clark SM, Shelp BJ (2008) γ-hydroxybutyrate accumulation in Arabidopsis and tobacco plants is a general response to abiotic stress: putative regulation by redox balance and glyoxylate reductase isoforms. J Exp Bot 59(9):2555–2564. doi:10.1093/jxb/ern122
Mirabella R, Rauwerda H, Struys EA, Jakobs C, Triantaphylides C, Haring MA, Schuurink RC (2008) The Arabidopsis her1 mutant implicates GABA in E-2-hexenal responsiveness. Plant J 53(2):197–213. doi:10.1111/j.1365-313X.2007.03323.x
Clark SM, Di Leo R, Dhanoa PK, Van Cauwenberghe OR, Mullen RT, Shelp BJ (2009) Biochemical characterization, mitochondrial localization, expression, and potential functions for an Arabidopsis gamma-aminobutyrate transaminase that utilizes both pyruvate and glyoxylate. J Exp Bot 60(6):1743–1757. doi:10.1093/jxb/erp044
Dimlioğlu G, Daş ZA, Bor M, Özdemir F, Türkan İ (2015) The impact of GABA in harpin-elicited biotic stress responses in Nicotiana tabaccum. J Plant Physiol 188:51–57
Aurisano N, Bertani A, Reggiani R (1995) Involvement of calcium and calmodulin in protein and amino acid metabolism in rice roots under anoxia. Plant Cell Physiol 36(8):1525–1529
Reggiani R, Cantu CA, Brambilla I, Bertani A (1988) Accumulation and interconversion of amino acids in rice roots under anoxia. Plant Cell Physiol 29(6):981–987
Kim DW, Shibato J, Agrawal GK, Fujihara S, Iwahashi H, du Kim H, Shim Ie S, Rakwal R (2007) Gene transcription in the leaves of rice undergoing salt-induced morphological changes (Oryza sativa L.). Mol Cells 24(1):45–59
Shimajiri Y, Oonishi T, Ozaki K, Kainou K, Akama K (2013) Genetic manipulation of the gamma-aminobutyric acid (GABA) shunt in rice: overexpression of truncated glutamate decarboxylase (GAD2) and knockdown of gamma-aminobutyric acid transaminase (GABA-T) lead to sustained and high levels of GABA accumulation in rice kernels. Plant Biotechnol J 11(5):594–604. doi:10.1111/pbi.12050
Liu L, Zhai H, Wan J-M (2005) Accumulation of γ-aminobutyric acid in giant-embryo rice grain in relation to glutamate decarboxylase activity and its gene expression during water soaking. Cereal Chem 82(2):191–196
Wallace W, Secor J, Schrader L (1984) Rapid accumulation of γ-aminobutyric acid and alanine in soybean leaves in response to an abrupt transfer to lower temperature, darkness, or mechanical manipulation. Plant Physiol 75:170–175
Ramputh A-I, Bown AW (1996) Rapid γ-aminobutyric acid synthesis and the inhibition of the growth and development of oblique-banded leaf-roller larvae. Plant Physiol 111(4):1349–1352
Akihiro T, Koike S, Tani R, Tominaga T, Watanabe S, Iijima Y, Aoki K, Shibata D, Ashihara H, Matsukura C (2008) Biochemical mechanism on GABA accumulation during fruit development in tomato. Plant Cell Physiol 49(9):1378–1389
Deewatthanawong R, Rowell P, Watkins CB (2010) γ-Aminobutyric acid (GABA) metabolism in CO2 treated tomatoes. Postharvest Biol Technol 57(2):97–105
Mae N, Makino Y, Oshita S, Kawagoe Y, Tanaka A, Aoki K, Kurabayashi A, Akihiro T, Akama K, Koike S, Takayama M, Matsukura C, Ezura H (2012) Accumulation mechanism of gamma-aminobutyric acid in tomatoes (Solanum lycopersicum L.) under low O2 with and without CO2. J Agric Food Chem 60(4):1013–1019. doi:10.1021/jf2046812
Bartyzel I, Pelczar K, Paszkowski A (2003) Functioning of the gamma-aminobutyrate pathway in wheat seedlings affected by osmotic stress. Biol Plantarum 47(2):221–225
C-y Wang, J-r Li, Xia Q-p Wu, X-l Gao H-b (2014) Influence of exogenous gamma-aminobutyric acid (GABA) on GABA metabolism and amino acid contents in roots of melon seedling under hypoxia stress. J Appl Ecology 25(7):2011–2018
Yang R, Chen H, Gu Z (2011) Factors influencing diamine oxidase activity and gamma-aminobutyric acid content of fava bean (Vicia faba L.) during germination. J Agric Food Chem 59(21):11616–11620. doi:10.1021/jf202645p
Martinez-Luscher J, Torres N, Hilbert G, Richard T, Sanchez-Diaz M, Delrot S, Aguirreolea J, Pascual I, Gomes E (2014) Ultraviolet-B radiation modifies the quantitative and qualitative profile of flavonoids and amino acids in grape berries. Phytochem 102:106–114. doi:10.1016/j.phytochem.2014.03.014
Allan W, Peiris C, Bown A, Shelp B (2003) Gamma-hydroxybutyrate accumulates in green tea and soybean sprouts in response to oxygen deficiency. Can J Plant Sci 83(4):951–953
Scharff AM, Egsgaard H, Hansen PE, Rosendahl L (2003) Exploring symbiotic nitrogen fixation and assimilation in pea root nodules by in vivo 15N nuclear magnetic resonance spectroscopy and liquid chromatography-mass spectrometry. Plant Physiol 131(1):367–378
Johnston G, Chebib M, Duke R, Fernandez S, Hanrahan J, Hinton T, Mewett K (2009) Herbal products and GABA receptors. Encycl Neurosci (4):1095-1101
Johnston GA (2005) GABAA receptor channel pharmacology. Curr Pharm Des 11(15):1867–1885
Johnston GA (1986) Multiplicity of GABA receptors. Benzodiazepine/GABA receptors and chloride channels: structural and functional properties
Ticku MK Drug modulation of GABAA-mediated transmission. In: Seminars in Neuroscience, 1991. vol 3. Elsevier, pp 211–218
Chebib M, Hanrahan JR, Mewett KN, Duke RK, Johnston GA (2004) Ionotropic GABA receptors as therapeutic targets for memory and sleep disorders. Annu Rep Med Chem 39:13–23
Viola H, Wasowski C, De Stein ML, Wolfman C, Silveira R, Dajas F, Medina J, Paladini A (1995) Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med 61(03):213–216
Patel D, Shukla S, Gupta S (2007) Apigenin and cancer chemoprevention: progress, potential and promise. Int J Oncol 30(1):233–246
Ruela-de-Sousa R, Fuhler G, Blom N, Ferreira C, Aoyama H, Peppelenbosch M (2010) Cytotoxicity of apigenin on leukemia cell lines: implications for prevention and therapy. Cell Death Dis 1(1):e19
Brogden RN, Goa KL (1991) Flumazenil. A reappraisal of its pharmacological properties and therapeutic efficacy as a benzodiazepine antagonist. Drugs 42(6):1061–1089
Spivey WH (1991) Flumazenil and seizures: analysis of 43 cases. Clin Ther 14(2):292–305
Kavvadias D, Sand P, Youdim KA, Qaiser MZ, Rice-Evans C, Baur R, Sigel E, Rausch WD, Riederer P, Schreier P (2004) The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood–brain barrier and exhibits anticonvulsive effects. Br J Pharmacol 142(5):811–820
Davidoff RA (1985) Antispasticity drugs: mechanisms of action. Ann Neurol 17(2):107–116
Bucknam W (2007) Suppression of symptoms of alcohol dependence and craving using high-dose baclofen. Alcohol Alcohol 42(2):158–160
Rando R (1977) Mechanism of the irreversible inhibition of γ-aminobutyric acid-α-ketoglutaric acid transaminase by the neurotoxin gabaculine. Biochem 16(21):4604–4610
Rando RR, Bangerter F (1977) The in vivo inhibition of GABA-transaminase by gabaculine. Biochem Biophys Res Commun 76(4):1276–1281
Ylinen A, Sivenius J, Pitkänen A, Halonen T, Partanen J, Mervaala E, Mumford J, Riekkinen P (1992) γ-Vinyl GABA (Vigabatrin) in Epilepsy: clinical, Neurochemical, and Neurophysiologic Monitoring in Epileptic Patients. Epilepsia 33(5):917–922
Connelly J (1993) Vigabatrin. Ann Pharmacother 27(2):197–204
Mathivet P, Bernasconi R, De Barry J, Marescaux C, Bittiger H (1997) Binding characteristics of γ-hydroxybutyric acid as a weak but selective GABAB receptor agonist. Eur J Pharmacol 321(1):67–75
Wong T, Guin C, Bottiglieri T, Snead OC (2003) GABA, γ-hydroxybutyric acid, and neurological disease. Ann Neurol 54(S6):S3–S12
Wong CGT, Gibson KM, Snead OC (2004) From the street to the brain: neurobiology of the recreational drug γ-hydroxybutyric acid. Trends Pharmacol Sci 25(1):29–34
Snead OC III, Gibson KM (2005) γ-Hydroxybutyric acid. N Engl J Med 352(26):2721–2732
Olsen RW (1981) GABA-benzodiazepine-barbiturate receptor interactions. J Neurochem 37(1):1–13
Olsen RW (1982) Drug interactions at the GABA receptor-ionophore complex. Annu Rev Pharmacol Toxicol 22(1):245–277
Olson R (1987) The gamma-aminobutyric acid/benzodiazepine/barbiturate receptor-chloride ion channel complex of the mammalian brain. Synaptic function. Wiley, New York
Haefely W, Kulcsar A, Möhler H, Pieri L, Polc P, Schaffner R (1974) Possible involvement of GABA in the central actions of benzodiazepines. Adv Biochem Psychopharmacol 14:131–151
Hunkeler W, Möhler H, Pieri L, Polc P, Bonetti E, Cumin R, Schaffner R, Haefely W (1981) Selective antagonists of benzodiazepines. Nature 290:514–516
Acknowledgements
Funding was provided by Centre of Excellence in Plant Energy Biology, Australian Research Council (Grant No. CE140100008) to S.D.T and M.G, and by Australian Research Council (Grant No. FT130100709) to M.G.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramesh, S.A., Tyerman, S.D., Gilliham, M. et al. γ-Aminobutyric acid (GABA) signalling in plants. Cell. Mol. Life Sci. 74, 1577–1603 (2017). https://doi.org/10.1007/s00018-016-2415-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2415-7