Abstract
An expanded polyglutamine (polyQ) tract at the amino-terminus of the androgen receptor (AR) confers toxic properties responsible for neuronal and non-neuronal degeneration in spinal and bulbar muscular atrophy (SBMA), one of nine polyQ expansion diseases. Both lower motor neurons and peripheral tissues, including skeletal muscle, are affected, supporting the notion that SBMA is not a pure motor neuron disease but a degenerative disorder of the neuromuscular system. Here, we review experimental evidence demonstrating both nerve and muscle degeneration in SBMA model systems and patients. We propose that polyQ AR toxicity targets these components in a time-dependent fashion, with muscle pathology predominating early and motor neuron loss becoming more significant at late stages. This model of pathogenesis has important therapeutic implications, suggesting that symptoms arising from degeneration of nerve or muscle predominate at different points and that directed interventions targeting these components will be variably effective depending upon disease progression.

Similar content being viewed by others
Abbreviations
- SBMA:
-
Spinal and bulbar muscular atrophy
- CAG:
-
Cytosine adenine guanine
- PolyQ:
-
Polyglutamine
- AR:
-
Androgen receptor
- T:
-
Testosterone
- DHT:
-
Dihydrotestosterone
- NTD:
-
Amino-terminal domain
- DBD:
-
DNA-binding domain
- LBD:
-
Ligand-binding domain
- AF-1/AF-2:
-
Activation function
- Tau-1/Tau-5:
-
Transcription activation unit
- SRC-1:
-
Steroid receptor coactivator-1
- NLS:
-
Nuclear localization signal
- PEST:
-
Proline, glutamic acid, serine, threonine
- CBP:
-
c-AMP responsive element binding protein
- Hsp:
-
Heat shock proteins
- CHIP:
-
Carboxyl terminus of Hsc70-interacting protein
- N/C interaction:
-
Amino- and carboxy-terminus interaction
- Lys:
-
Lysine
- Ser:
-
Serine
- SIRT1:
-
Sirtuin 1
- IGF-1:
-
Insulin-like growth factor-1
- CNS:
-
Central nervous system
References
Orr HT, Zoghbi HY (2007) Trinucleotide repeat disorders. Annu Rev Neurosci 30:575–621
La Spada AR et al (1991) Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352(6330):77–79
Kawahara H (1897) A family of progressive bulbar palsy. Aichi Med School J 16:3–4
Kennedy W, Alter M, Sung J (1968) Progressive proximal spinal and bulbar muscular atrophy of late onset. Neurology 18(7):671–680
Harding AE et al (1982) X-linked recessive bulbospinal neuronopathy: a report of ten cases. J Neurol Neurosurg Psychiatry 45(11):1012–1019
Schmidt BJ et al (2002) Expression of X-linked bulbospinal muscular atrophy (Kennedy disease) in two homozygous women. Neurology 59(5):770–772
Sobue G et al (1993) Subclinical phenotypic expressions in heterozygous females of X-linked recessive bulbospinal neuronopathy. J Neurol Sci 117(1–2):74–78
Nagashima T et al (1988) Familial bulbo-spinal muscular atrophy associated with testicular atrophy and sensory neuropathy (Kennedy–Alter–Sung syndrome). Autopsy case report of two brothers. J Neurol Sci 87(2–3):141–152
Battaglia F et al (2003) Kennedy’s disease initially manifesting as an endocrine disorder. J Clin Neuromuscul Dis 4(4):165–167
Yu Z et al (2006) Androgen-dependent pathology demonstrates myopathic contribution to the Kennedy disease phenotype in a mouse knock-in model. J Clin Invest 116(10):2663–2672
Chevalier-Larsen ES et al (2004) Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy. J Neurosci 24(20):4778–4786
Katsuno M et al (2002) Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron 35(5):843–854
Fischbeck K et al (1986) Localization of the gene for X-linked spinal muscular atrophy. Neurology 36(12):1595–1598
Poletti A (2004) The polyglutamine tract of androgen receptor: from functions to dysfunctions in motor neurons. Front Neuroendocrinol 25:1–26
Clark P, Irvine R, Coetzee G (2003) The androgen receptor CAG repeat and prostate cancer risk. Methods Mol Med 81:255–266
Davis-Dao C et al (2012) Shorter androgen receptor CAG repeat lengths associated with cryptorchidism risk among Hispanic white boys. J Clin Endocrinol Metab 97(3):E393–E399
Palazzolo I et al (2008) The role of the polyglutamine tract in androgen receptor. J Steroid Biochem Mol Biol 108(3–5):245–253
Chang C et al (1995) Androgen receptor: an overview. Crit Rev Eukaryot Gene Expr 5(2):97–125
Callewaert L, Van Tilborgh N, Claessens F (2006) Interplay between two hormone-independent activation domains in the androgen receptor. Cancer Res 66(1):543–553
Tan EM et al (2015) Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin 36(1):3–23
Christiaens V et al (2002) Characterization of the two coactivator-interacting surfaces of the androgen receptor and their relative role in transcriptional control. J Biol Chem 277(51):49230–49237
Callewaert L et al (2003) Implications of a polyglutamine tract in the function of the human androgen receptor. Biochem Biophys Res Commun 306(1):46–52
Bevan CL et al (1999) The AF1 and AF2 domains of the androgen receptor interact with distinct regions of SRC1. Mol Cell Biol 19(12):8383–8392
McCampbell A et al (2000) CREB-binding protein sequestration by expanded polyglutamine. Hum Mol Genet 9(4):2197–2202
Stenoien DL et al (1999) Polyglutamine-expanded androgen receptors form aggregates that sequester heat shock proteins, proteasome components and SRC-1, and are suppressed by the HDJ-2 chaperone. Hum Mol Genet 8(5):731–741
Rechsteiner M, Rogers SW (1996) PEST sequences and regulation by proteolysis. Trends Biochem Sci 21(6):267–271
Ni L et al (2013) Androgen induces a switch from cytoplasmic retention to nuclear import of the androgen receptor. Mol Cell Biol 33(24):4766–4778
Tanner TM et al (2010) A 629RKLKK633 motif in the hinge region controls the androgen receptor at multiple levels. Cell Mol Life Sci 67(11):1919–1927
Haelens A et al (2007) The hinge region regulates DNA binding, nuclear translocation, and transactivation of the androgen receptor. Cancer Res 67(9):4514–4523
Clinckemalie L et al (2012) The hinge region in androgen receptor control. Mol Cell Endocrinol 358(1):1–8
Fu M et al (2000) p300 and p300/cAMP-response element-binding protein-associated factor acetylate the androgen receptor at sites governing hormone-dependent transactivation. J Biol Chem 275(27):20853–20860
Katsuno M et al (2012) Pathogenesis and therapy of spinal and bulbar muscular atrophy (SBMA). Prog Neurobiol 99(3):246–256
He B et al (1999) Activation function 2 in the human androgen receptor ligand binding domain mediates interdomain communication with the NH(2)-terminal domain. J Biol Chem 274(52):37219–37225
Saporita AJ et al (2003) Identification and characterization of a ligand-regulated nuclear export signal in androgen receptor. J Biol Chem 278(43):41998–42005
Kratter IH, Finkbeiner S (2010) PolyQ disease: too many Qs, too much function? Neuron 67(6):897–899
Lieberman AP et al (2014) Peripheral androgen receptor gene suppression rescues disease in mouse models of spinal and bulbar muscular atrophy. Cell Rep 7(3):774–784
Cortes CJ et al (2014) Muscle expression of mutant androgen receptor accounts for systemic and motor neuron disease phenotypes in spinal and bulbar muscular atrophy. Neuron 82(2):295–307
Adachi H et al (2005) Widespread nuclear and cytoplasmic accumulation of mutant androgen receptor in SBMA patients. Brain 128(3):659–670
Yu Z et al (2006) Abnormalities of germ cell maturation and sertoli cell cytoskeleton in androgen receptor 113 CAG knock-in mice reveal toxic effects of the mutant protein. Am J Pathol 168(1):195–204
Wyttenbach A (2004) Role of heat shock proteins during polyglutamine neurodegeneration. J Mol Neurosci 23(1–2):69–95
Jochum T et al (2012) Toxic and non-toxic aggregates from the SBMA and normal forms of androgen receptor have distinct oligomeric structures. Biochim Biophys Acta 1822(6):1070–1078
Taylor JP et al (2003) Aggresomes protect cells by enhancing the degradation of toxic polyglutamine-containing protein. Hum Mol Genet 12(7):749–757
Muchowski PJ, Wacker JL (2005) Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci 6(1):11–22
Li M et al (1998) Nonneural nuclear inclusions of androgen receptor protein in spinal and bulbar muscular atrophy. Am J Pathol 153(3):695–701
Li M et al (1998) Nuclear inclusions of the androgen receptor protein in spinal and bulbar muscular atrophy. Ann Neurol 44(2):249–254
Miller J et al (2011) Identifying polyglutamine protein species in situ that best predict neurodegeneration. Nat Chem Biol 7(12):925–934
Montie HL et al (2009) Cytoplasmic retention of polyglutamine-expanded androgen receptor ameliorates disease via autophagy in a mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet 18(11)
Takeyama K-I et al (2002) Androgen-dependent neurodegeneration by polyglutamine-expanded human androgen receptor in Drosophila. Neuron 35(5):855–864
Pratt WB, Toft DO (2003) Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood)
Pratt WB et al (2014) A model in which heat shock protein 90 targets protein-folding clefts: rationale for a new approach to neuroprotective treatment of protein folding diseases. Exp Biol Med 1–9
Pratt WB et al (2010) Proposal for a role of the Hsp90/Hsp70-based chaperone machinery in making triage decisions when proteins undergo oxidative and toxic damage. Exp Biol Med (Maywood) 235(3):278–288
Pratt WB et al (2014) Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annu Rev Pharmacol Toxicol 55:353–371
Pratt WB, Morishima Y, Osawa Y (2008) The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts. J Biol Chem 283(34):22885–22889
Adachi H et al (2007) CHIP overexpression reduces mutant androgen receptor protein and ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model. Neurobiol Dis 27(19):5115–5126
Waza M et al (2005) 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat Med 11:1088–1095
Wang AM et al (2013) Activation of Hsp70 reduces neurotoxicity by promoting polyglutamine protein degradation. Nat Chem Biol 9:112–118
Van Royen ME et al (2012) Stepwise androgen receptor dimerization. J Cell Sci 125(8):1970–1979
Nedelsky NB et al (2010) Native functions of the androgen receptor are essential to pathogenesis in a Drosophila model of spinobulbar muscular atrophy. Neuron 67(6):936–952
Orr CR et al (2010) An interdomain interaction of the androgen receptor is required for its aggregation and toxicity in spinal and bulbar muscular atrophy. J Biol Chem 285(46):35567–35577
Zboray L et al (2015) Preventing the androgen receptor N/C interaction delays disease onset in a mouse model of SBMA. Cell Rep 13(10):2312–2323
Lieberman AP et al (2002) Altered transcriptional regulation in cells expressing the expanded polyglutamine androgen receptor. Hum Mol Genet 11(17):1967–1976
Powell SM et al (2004) Mechanisms of androgen receptor signalling via steroid receptor coactivator-1 in prostate. Endocr Relat Cancer 11(1):117–130
Katsuno M et al (2010) Disrupted transforming growth factor-beta signaling in spinal and bulbar muscular atrophy. J Neurosci 30(16):5702–5712
Sopher B et al (2004) Androgen receptor YAC transgenic mice recapitulate SBMA motor neuronopathy and implicate VEGF164 in the motor neuron degeneration. Neuron 41(5):687–699
Minamiyama M et al (2004) Sodium butyrate ameliorates phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet 13(11):1183–1192
Butler R, Bates GP (2006) Histone deacetylase inhibitors as therapeutics for polyglutamine disorders. Nat Rev Neurosci 7(10):784–796
McCampbell A et al (2001) Histone deacetylase inhibitors reduce polyglutamine toxicity. Proc Natl Acad Sci USA 98(26):15179–15184
Steffan JS et al (2001) Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413(6857):739–743
Palazzolo I et al (2007) Akt blocks ligand binding and protects against expanded polyglutamine androgen receptor toxicity. Hum Mol Genet 16(13):1593–1603
Palazzolo I et al (2009) Overexpression of IGF-1 in muscle attenuates disease in a mouse model of spinal and bulbar muscular atrophy. Neuron 63(3):316–328
Montie HL, Pestell RG, Merry DE (2011) SIRT1 modulates aggregation and toxicity through deacetylation of the androgen receptor in cell models of SBMA. Neurobiol Dis 31(48):17425–17436
Mukherjee S et al (2009) Small ubiquitin-like modifier (SUMO) modification of the androgen receptor attenuates polyglutamine-mediated aggregation. J Biol Chem 284(32):21296–21306
Chua JP et al (2014) Disrupting SUMOylation potentiates transactivation function and ameliorates polyglutamine AR-mediated disease. J Clin Invest
Sobue G et al (1989) X-linked recessive bulbospinal neuronopathy. A clinicopathological study. Brain 112:209–232
Suzuki K et al (2008) CAG repeat size correlates to electrophysiological motor and sensory phenotypes in SBMA. Brain 131(1):229–239
Katsuno M et al (2006) Pathogenesis, animal models and therapeutics in spinal and bulbar muscular atrophy (SBMA). Exp Neurol 200(1):8–18
Suzuki K et al (2010) The profile of motor unit number estimation (MUNE) in spinal and bulbar muscular atrophy. J Neurol Neurosurg Psychiatry 81(5):567–571
Atsuta N et al (2006) Natural history of spinal and bulbar muscular atrophy (SBMA): a study of 223 Japanese patients. Brain 129(6):1446–1455
Rhodes LE et al (2009) Clinical features of spinal and bulbar muscular atrophy. Brain 132(12):3242–3251
Sorarù G et al (2008) Spinal and bulbar muscular atrophy: skeletal muscle pathology in male patients and heterozygous females. J Neurol Sci 264(1–2):100–105
Chua JP et al (2014) Transcriptional activation of TFEB/ZKSCAN3 target genes underlies enhanced autophagy in spinobulbar muscular atrophy. Hum Mol Genet 23(5):1376–1386
Yu Z et al (2011) Macroautophagy is regulated by the UPR-mediator CHOP and accentuates the phenotype of SBMA mice. PLoS Genet 7(10)
Rusmini P et al (2015) Aberrant autophagic response in the muscle of a knock-in mouse model of spinal and bulbar muscular atrophy. Sci Rep 5(15174)
Monks DA et al (2007) Overexpression of wild-type androgen receptor in muscle recapitulates polyglutamine disease. Proc Natl Acad Sci USA 104(46):18259–18264
Rinaldi C et al (2012) Insulin like growth factor (IGF)-1 administration ameliorates disease manifestations in a mouse model of spinal and bulbar muscular atrophy. Mol Med 18(1):1261–1268
Malena A et al (2013) Androgen-dependent impairment of myogenesis in spinal and bulbar muscular atrophy. Acta Neuropathol 126:109–121
Funakoshi H et al (1993) Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol 123(2):455–465
Ramzan F et al (2015) Distinct etiological roles for myocytes and motor neurons in a mouse model of Kennedy’s disease/spinobulbar muscular atrophy. J Neurosci 35(16):6444–6451
Sahashi K et al (2015) Silencing neuronal mutant androgen receptor in a mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet 24(21):5985–5994
Acknowledgments
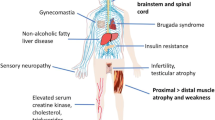
We thank Kayla Capper for help creating the illustration. Supported by the National Institutes of Health (R01 NS055746, R21 NS089516 to A.P.L.) and by the University of Michigan Protein Folding Disease Initiative.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giorgetti, E., Lieberman, A.P. Polyglutamine androgen receptor-mediated neuromuscular disease. Cell. Mol. Life Sci. 73, 3991–3999 (2016). https://doi.org/10.1007/s00018-016-2275-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2275-1