Abstract
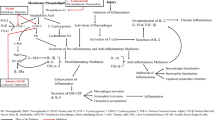
Inflammation is a complex, metabolically expensive process involving multiple signaling pathways and regulatory mechanisms which have evolved over evolutionary timescale. Addressing multiple targets of inflammation holistically, in moderation, is probably a more evolutionarily viable strategy, as compared to current therapy which addresses drug targets in isolation. Polypharmacology, addressing multiple targets, is commonly used in complex ailments, suggesting the superior safety and efficacy profile of multi-target (MT) drugs. Phenotypic drug discovery, which generated successful MT and first-in-class drugs in the past, is now re-emerging. A multi-pronged approach, which modulates the evolutionarily conserved, robust and pervasive cellular mechanisms of tissue repair, with AMPK at the helm, regulating the complex metabolic/immune/redox pathways underlying inflammation, is perhaps a more viable strategy than addressing single targets in isolation. Molecules that modulate multiple molecular mechanisms of inflammation in moderation (modulating TH cells toward the anti-inflammatory phenotype, activating AMPK, stimulating Nrf2 and inhibiting NFκB) might serve as a model for a novel Darwinian “first-in-class” therapeutic category that holistically addresses immune, redox and metabolic processes associated with inflammatory repair. Such a multimodal biological activity is supported by the fact that several non-calorific pleiotropic natural products with anti-inflammatory action have been incorporated into diet (chiefly guided by the adaptive development of olfacto-gustatory preferences over evolutionary timescales) rendering such molecules, endowed with evolutionarily privileged molecular scaffolds, naturally oriented toward multiple targets.
Similar content being viewed by others
References
Medina-Franco JL, Giulianotti MA, Welmaker GS, Houghten RA. Shifting from the single to the multitarget paradigm in drug discovery. Drug Discov Today. 2013;18:495–501.
Zimmermann GR, Lehar J, Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007;12:34–42.
Beejay U, Wolfe M. COX2-selective inhibitors : panacea or flash in the pan. Gastroenterology. 1999;117:1002–5.
Unnikrishnan MK, Veerapur V, Nayak Y, Paul P, Mathew G. Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoids. In: Watson RR, Preedy VR, Zibadi S, editors. Polyphenols in human health and disease. UK: Elsevier; 2014. p. 143–61.
Swinney DC. The value of translational biomarkers to phenotypic assays. Front Pharmacol. 2014;5:171.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77.
Lounkine E, Kutchukian P, Petrone P, Davies JW, Glick M. Chemotography for multi-target SAR analysis in the context of biological pathways. Bioorg Med Chem. 2012;20:5416–27.
Kotz J. Phenotypic screening, take two. Sci Bus Exch. 2012;5(15):1–3.
Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov. 2011;10:507–19.
Eder J, Sedrani R, Wiesmann C. The discovery of first-in-class drugs: origins and evolution. Nat Rev Drug Discov. 2014;13:577–87.
Swinney DC. Phenotypic vs. target-based drug discovery for first-in-class medicines. Clin Pharmacol Ther. 2013;93:299–301.
Zheng W, Thorne N, McKew JC. Phenotypic screens as a renewed approach for drug discovery. Drug Discov Today. 2013;18:1067–73.
Morphy JR, Harris CJ. Designing multi-target drugs. Cambridge: Royal Society of Chemistry; 2012. p. P011–4.
Lu JJ, Pan W, Hu YJ, Wang YT. Multi-target drugs: the trend of drug research and development. PLoS One. 2012;7:e40262.
Bolognesi ML. Polypharmacology in a single drug: multitarget drugs. Curr Med Chem. 2013;20:1639–45.
Csermely P, Agoston V, Pongor S. The efficiency of multi-target drugs: the network approach might help drug design. Trends Pharmacol Sci. 2005;26:178–82.
Singhal A, Jie L, Kumar P, Hong GS, Leow MK-S, Paleja B, et al. Metformin as adjunct antituberculosis therapy. Sci Transl Med. 2014;6:263ra159.
Morsy MA, Ashour OM, Fouad AA, Abdel-Gaber SA. Gastroprotective effects of the insulin sensitizers rosiglitazone and metformin against indomethacin-induced gastric ulcers in Type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2010;37:173–7.
Kaplan MJ. Cardiovascular complications of rheumatoid arthritis: assessment, prevention, and treatment. Rheum Dis Clin North Am. 2010;36:405–26.
Martin-Martinez MA, Gonzalez-Juanatey C, Castaneda S, Llorca J, Ferraz-Amaro I, Fernandez-Gutierrez B, et al. Recommendations for the management of cardiovascular risk in patients with rheumatoid arthritis: scientific evidence and expert opinion. Semin Arthritis Rheum. 2014;44:1–8.
Hardie DG. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes. 2013;62:2164–72.
Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9.
Mor V, Unnikrishnan M. 5′-Adenosine Monophosphate-Activated Protein Kinase and the Metabolic Syndrome. Endocr Metab Immune Disord Drug Targets. 2011;11:206–16.
Mathew G, Thambi M, Unnikrishnan MK. A multimodal Darwinian strategy for alleviating the atherosclerosis pandemic. Med Hypotheses. 2014;82:159–62.
Surh YJ. NF-kappa B and Nrf2 as potential chemopreventive targets of some anti-inflammatory and antioxidative phytonutrients with anti-inflammatory and antioxidative activities. Asia Pac J Clin Nutr. 2008;17(Suppl 1):269–72.
O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–55.
Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11:230–41.
Gerriets VA, Rathmell JC. Metabolic pathways in T cell fate and function. Trends Immunol. 2012;33:168–73.
Bai A, Ma AG, Yong M, Weiss CR, Ma Y, Guan Q, et al. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem Pharmacol. 2010;80:1708–17.
Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, et al. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin J(2). Mol Cell Biol. 2004;24:36–45.
Mo C, Wang L, Zhang J, Numazawa S, Tang H, Tang X, et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. 2014;20:574–88.
Mathew G. Pharmacological, mechanistic and in silico evaluation of selected novel non-ulcerogenic anti-inflammatory indanedione derivatives. In: Ph.D. Dissertation. Manipal: Manipal University; 2014.
Weiss U. Inflammation. Nature. 2008;454:427.
Spiegel DA. Synthetic immunology to engineer human immunity. Nat Chem Biol. 2010;6:871–2.
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16.
Yang X, Gao X. Role of dendritic cells: a step forward for the hygiene hypothesis. Cell Mol Immunol. 2011;8:12–8.
Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462:449–60.
Motta AC, Vissers JLM, Gras R, Van Esch BCAM, Van Oosterhout AJM, Nawijn MC. GITR signaling potentiates airway hyperresponsiveness by enhancing Th2 cell activity in a mouse model of asthma. Resp Res. 2009;10:93.
Peck A, Mellins ED. Precarious balance: Th17 Cells in host defense. Infect Immun. 2010;78:32–8.
Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–69.
Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–5.
Galgani M, Di Giacomo A, Matarese G, La Cava A. The Yin and Yang of CD4(+) regulatory T cells in autoimmunity and cancer. Curr Med Chem. 2009;16:4626–31.
Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage review with regulatory T cell ties. Immunity. 2006;24:677–88.
Lee EY, Abbondante S. Tissue-specific tumor suppression by BRCA1. Proc Natl Acad Sci USA. 2014;111:4353–4.
Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–107.
Kim H, Jung Y, Shin BS, Song H, Bae SH, Rhee SG, et al. Redox regulation of lipopolysaccharide-mediated sulfiredoxin induction, which depends on both AP-1 and Nrf2. J Biol Chem. 2010;285:34419–28.
Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74:1526–39.
Ueda K, Ueyama T, K-i Yoshida, Kimura H, Ito T, Shimizu Y, et al. Adaptive HNE-Nrf2-HO-1 pathway against oxidative stress is associated with acute gastric mucosal lesions. Am J Physiol Gastrointest Liver Physiol. 2008;295:G460–9.
Hahm KB, Lee HJ, Han Y, Kim EH, Kim YJ. A possible involvement of Nrf2-mediated heme oxygenase-1 up-regulation in protective effect of the proton pump inhibitor pantoprazole against indomethacin-induced gastric damage in rats. BMC Gastroenterol. 2012;12:143.
Buommino E, Donnarumma G, Manente L, De Filippis A, Silvestri F, Iaquinto S, et al. The Helicobacter pylori protein HspB interferes with Nrf2/Keap1 pathway altering the antioxidant response of Ags cells. Helicobacter. 2012;17:417–25.
Williams M, Rangasamy T, Bauer S, Killedar S, Karp M, Kensler T, et al. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol. 2008;181:4545–59.
Rockwell CE, Zhang M, Fields PE, Klaassen CD. Th2 skewing by activation of Nrf2 in CD4(+) T cells. J Immunol. 2012;188:1630–7.
Jiang X, Liu X, Greiner J, Szucs SS, Visnick M. Antioxidant inflammation modulators: C-17 homologated oleanolic acid derivatives. Google Patents; 2011.
Crunkhorn S. Deal watch: abbott boosts investment in NRF2 activators for reducing oxidative stress. Nat Rev Drug Discov. 2012;11:96.
Zhu M, Fu Y. The complicated role of NF-kappaB in T-cell selection. Cell Mol Immunol. 2010;7:89–93.
Mortaz E, Redegeld FA, Nijkamp FP, Engels F. Dual effects of acetyl salicylic acid on mast cell degranulation, expression of COX-2 and release of inflammatory cytokines. Biochem Pharmacol. 2005;69:1049–57.
Tomita M, Irwin KI, Xie ZJ, Santoro TJ. Tea pigments inhibit the production of type 1 (T(H1)) and type 2 (T(H2)) helper T cell cytokines in CD4(+) T cells. Phytother Res. 2002;16:36–42.
Surh Y-J, Kundu JK, Na H-K, Lee J-S. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutri. 2005;135:2993S–3001S.
Yu M, Li H, Liu Q, Liu F, Tang L, Li C, et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell Signal. 2011;23:883–92.
Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89:1025–78.
Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418:261–75.
Steinberg GR, Schertzer JD. AMPK promotes macrophage fatty acid oxidative metabolism to mitigate inflammation: implications for diabetes and cardiovascular disease. Immunol Cell Biol. 2014;92:340–5.
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+T cell subsets. J Immunol. 2011;186:3299–303.
Michalek RD, Gerriets VA, Nichols AG, Inoue M, Kazmin D, Chang CY, et al. Estrogen-related receptor-alpha is a metabolic regulator of effector T-cell activation and differentiation. Proc Natl Acad Sci USA. 2011;108:18348–53.
Dong Y, Zhang M, Liang B, Xie Z, Zhao Z, Asfa S, et al. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 2010;121:792–803.
Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, et al. Metformin inhibits proinflammatory responses and nuclear factor-b in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26:611–7.
Bai A, Yong M, Ma AG, Ma Y, Weiss CR, Guan Q, et al. Novel anti-inflammatory action of 5-aminoimidazole-4-carboxamide ribonucleoside with protective effect in dextran sulfate sodium-induced acute and chronic colitis. J Pharmacol Exp Ther. 2010;333:717–25.
Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–22.
Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–8.
Peairs A, Radjavi A, Davis S, Li L, Ahmed A, Giri S, et al. Activation of AMPK inhibits inflammation in MRL/lpr mouse mesangial cells. Clin Exp Immunol. 2009;156:542–51.
Li XN, Song J, Zhang L, LeMaire SA, Hou X, Zhang C, et al. Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes. 2009;58:2246–57.
Cordain L. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–54.
Zhang H-Y, Chen L-L, Li X-J, Zhang J. Evolutionary inspirations for drug discovery. Trends Pharmacol Sci. 2010;31:443–8.
Zahorik DM. Associative and non-associative factors in learned food preferences. In: Barker LM, Best MR, Domjan M, editors. Learning mechanisms in food selection. Waco: Baylor University Press; 1977.
Zahorik DM, Maier SF, Pies RW. Preferences for tastes paired with recovery from thiamine deficiency in rats: appetitive conditioning or learned safety. J Comp Physiol Psychology. 1974;87:1083–91.
Revusky S. The concurrent interference approach to delay learning. In: Barker LM, Best MR, Domjan M, editors. Learning mechanisms in food selection. Waco: Baylor University Press; 1977.
Acknowledgments
This work was partly supported by Govt. of India, Department of Science and Technology—Inspire Fellowship awarded to GM and Manipal University, Dr. T.M.A Pai Endowment Chair in Cognition awarded to MKU.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Mathew, G., Unnikrishnan, M.K. Multi-target drugs to address multiple checkpoints in complex inflammatory pathologies: evolutionary cues for novel “first-in-class” anti-inflammatory drug candidates: a reviewer’s perspective. Inflamm. Res. 64, 747–752 (2015). https://doi.org/10.1007/s00011-015-0851-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0851-8