Abstract
Objective
To determine the optimal intervention time of the vagal stimulation (VS) attenuating myocardial ischemia/reperfusion injury (IRI).
Methods
One hundred and twenty male SD rats were randomly allocated into six groups: sham group, IRI group, the VS performed at 15 min of ischemia (VSI15) group, the VS performed immediately before reperfusion (VSR0) group, the VS performed at 30 min of reperfusion (VSR30) group, and the VS performed at 60 min of reperfusion (VSR60) group. Rats in each group were further allocated into subgroups A and B. In each group, the hemodynamics and ventricular arrhythmias were continuously observed. In the subgroup A, serum inflammatory cytokine levels were tested, and infarct size was assessed. In the subgroup B, myocardial inflammatory cytokine levels in both ischemic and non-ischemic regions were assayed.
Results
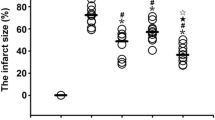
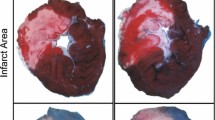
As compared to the IRI, VSR0, VSR30 and VSR60 groups, infarct size, serum HMGB-1 and ICAM-1 levels at 120 min of reperfusion, myocardial HMGB-1, IL-1 and IL-6 levels in non-ischemic region, myocardial ICAM-1 level in ischemic region were all significantly decreased in the VSI15 group. Compared with the IRI group, myocardial IL-10 levels in both ischemic and non-ischemic regions were significantly increased in the VSI15 group. Compared to the IRI, VSR0, VSR30 and VSR60 groups, incidence and score of ventricular arrhythmia during initial reperfusion were significantly decreased in the VSI15 group.
Conclusions
The VS performed at 15 min of ischemia provides the best protection against myocardial IRI. Also, early modulation on inflammatory responses caused by myocardial IRI may contribute to this best cardioprotection.




Similar content being viewed by others
References
Miller TD, Christian TF, Hopfenspirger MR, Hodge DO, Gersh BJ, Gibbons RJ. Infarct size after acute myocardial infarction measured by quantitative tomographic 99mTc sestamibi imaging predicts subsequent mortality. Circulation. 1995;92(3):334–41.
Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–35.
Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361(9351):13–20.
Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53(1):31–47.
Steffens S, Montecucco F, Mach F. The inflammatory response as a target to reduce myocardial ischaemia and reperfusion injury. Thromb Haemost. 2009;102(2):240–7.
Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123(6):594–604.
Wang Q, Cheng Y, Xue FS, Yuan YJ, Xiong J, Li RP, Liao X, Liu JH. Postconditioning with vagal stimulation attenuates local and systemic inflammatory responses to myocardial ischemia reperfusion injury in rats. Inflamm Res. 2012;61(11):1273–82.
Uemura K, Zheng C, Li M, Kawada T, Sugimachi M. Early short-term vagal nerve stimulation attenuates cardiac remodeling after reperfused myocardial infarction. J Card Fail. 2010;16(8):689–99.
Calvillo L, Vanoli E, Andreoli E, Besana A, Omodeo E, Gnecchi M, Zerbi P, Vago G, Busca G, Schwartz PJ. Vagal stimulation, through its nicotinic action, limits infarct size and the inflammatory response to myocardial ischemia and reperfusion. J Cardiovasc Pharmacol. 2011;58(5):500–7.
Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9(6):418–28.
Zitnik RJ. Treatment of chronic inflammatory diseases with implantable medical devices. Ann Rheum Dis. 2011;70(Suppl 1):i67–70.
Howland RH, Shutt LS, Berman SR, Spotts CR, Denko T. The emerging use of technology for the treatment of depression and other neuropsychiatric disorders. Ann Clin Psychiatry. 2011;23(1):48–62.
Shuchman M. Approving the vagus-nerve stimulator for depression. N Engl J Med. 2007;356(16):1604–7.
Zhang JQ, Wang Q, Xue FS, Li RP, Cheng Y, Cui XL, Liao X, Meng FM. Ischemic preconditioning produces more powerful anti-inflammatory and cardioprotective effects than limb remote ischemic postconditioning in rats with myocardial ischemia-reperfusion injury. Chin Med J. 2013;126(20):3949–55.
Stumpner J, Redel A, Kellermann A, Lotz CA, Blomeyer CA, Smul TM, Kehl F, Roewer N, Lange M. Differential role of Pim-1 kinase in anesthetic-induced and ischemic preconditioning against myocardial infarction. Anesthesiology. 2009;111(6):1257–64.
Kakinuma Y, Ando M, Kuwabara M, Katare RG, Okudela K, Kobayashi M, Sato T. Acetylcholine from vagal stimulation protects cardiomyocytes against ischemia and hypoxia involving additive non-hypoxic induction of HIF-1α. FEBS Lett. 2005;579(10):2111–8.
Ravingerová T, Pancza D, Ziegelholffer A, Styk J. Preconditioning modulates susceptibility to ischemia-induced arrhythmias in the rat heart: The role of α-adrenergic stimulation and K +ATP channels. Physiol Res. 2002;51(2):109–19.
Kawada T, Yamazaki T, Akiyama T, Kitagawa H, Shimizu S, Mizuno M, Li M, Sugimachi M. Vagal stimulation suppresses ischemia-induced myocardial interstitial myoglobin release. Life Sci. 2008;83(13–14):490–5.
Dai AL, Fan LH, Zhang FJ, Yang MJ, Yu J, Wang JK, Fang T, Chen G, Yu LN, Yan M. Effects of sevoflurane preconditioning and postconditioning on rat myocardial stunning in ischemic reperfusion injury. J Zhejiang Univ Sci B. 2010;11(4):267–74.
Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98(21):2334–51.
Zuanetti G, De Ferrari GM, Priori SG, Schwartz PJ. Protective effect of vagal stimulation on reperfusion arrhythmias in cats. Circ Res. 1987;61(3):429–35.
Mioni C, Bazzani C, Giuliani D, Altavilla D, Leone S, Ferrari A, Minutoli L, Bitto A, Marini H, Zaffe D, Botticelli AR, Iannone A, Tomasi A, Bigiani A, Bertolini A, Squadrito F, Guarini S. Activation of an efferent cholinergic pathway produces strong protection against myocardial ischemia/reperfusion injury in rats. Crit Care Med. 2005;33(11):2621–8.
Ando M, Katare RG, Kakinuma Y, Zhang D, Yamasaki F, Muramoto K, Sato T. Efferent vagal nerve stimulation protects heart against ischemia-induced arrhythmias by preserving connexin43 protein. Circulation. 2005;112(2):164–70.
Pourkhalili K, Hajizadeh S, Tiraihi T, Akbari Z, Esmailidehaj M, Bigdeli MR, Khoshbaten A. Ischemia and reperfusion-induced arrhythmias: role of hyperoxic preconditioning. J Cardiovasc Med (Hagerstown). 2009;10(8):635–42.
Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbäurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117(25):3216–26.
Xiong J, Xue FS, Yuan YJ, Wang Q, Liao X, Wang WL. Cholinergic anti-inflammatory pathway: a possible approach to protect against myocardial ischemia reperfusion injury. Chin Med J. 2010;123(19):2720–6.
Martelli D, McKinley MJ, McAllen RM. The cholinergic anti-inflammatory pathway: a critical review. Auton Neurosci. 2014;182:65–9.
Lataro RM, Silva CA, Fazan R Jr, Rossi MA, Prado CM, Godinho RO, Salgado HC. Increase in parasympathetic tone by pyridostigmine prevents ventricular dysfunction during the onset of heart failure. Am J Physiol Regul Integr Physiol. 2013;305(8):R908–16.
Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, Rosas-Ballina M, Ashok M, Goldstein RS, Chavan S, Pavlov VA, Metz CN, Yang H, Czura CJ, Wang H, Tracey KJ. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35(12):2762–8.
Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21(5):331–44.
Yang Z, Zingarelli B, Szabo C. Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation. 2000;101(9):1019–26.
Pasqui AL, Di Renzo M, Maffei S, Pastorelli M, Pompella G, Auteri A, Puccetti L. Pro/Anti-inflammatory cytokine imbalance in postischemic left ventricular remodeling. Mediat Inflamm. 2010;2010:974694.
Velasco CE, Turner M, Inagami T, Atkinson JB, Virmani R, Jackson EK, Murray JJ, Forman MB. Reperfusion enhances the local release of endothelin after regional myocardial ischemia. Am Heart J. 1994;128(3):441–51.
Engler RL, Dahlgren MD, Morris DD, Peterson MA, Schmid-Schönbein GW. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol. 1986;251(2Pt 2):H314–23.
Babior BM. The respiratory burst of phagocytes. J Clin Invest. 1984;73(3):599–601.
Abdel-Rahman U, Margraf S, Aybek T, Lögters T, Bitu-Moreno J, Francischetti I, Kranert T, Grünwald F, Windolf J, Moritz A, Scholz M. Inhibition of neutrophil activity improves cardiac function after cardiopulmonary bypass. J Inflamm Lond. 2007;4:21.
Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature. 1997;385(6618):729–33.
Sack MN, Smith RM, Opie LH. Tumor necrosis factor in myocardial hypertrophy and ischaemia-an anti-apoptotic perspective. Cardiovasc Res. 2000;45(3):688–95.
Kimura H, Shintani-Ishida K, Nakajima M, Liu S, Matsumoto K, Yoshida K. Ischemic preconditioning or p38 MAP kinase inhibition attenuates myocardial TNF α production and mitochondria damage in brief myocardial ischemia. Life Sci. 2006;78(17):1901–10.
Park SY, Kim DJ, Aldohayan A, Ahmed I, Husain S. Al Rikabi A, Aldawlatly A, Al Obied O, Hajjar W, Al Nassar S. Immune response after systematic lymph node dissection in lung cancer surgery: changes of interleukin-6 level in serum, pleural lavage fluid, and lung supernatant in a dog model. World J Surg Oncol. 2013;11:270.
Li J, Mathieu SL, Harris R, Ji J, Anderson DJ, Malysz J, Bunnelle WH, Waring JF, Marsh KC, Murtaza A, Olson LM, Gopalakrishnan M. Role of α7 nicotinic acetylcholine receptors in regulating tumor necrosis factor-α (TNF-α) as revealed by subtype selective agonists. J Neuroimmunol. 2011;239(1–2):37–43.
Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110(1):159–73.
Kawada T, Yamazaki T, Akiyama T. Vagal stimulation suppresses ischemia-induced myocardial interstitial norepinephrine release. Life Sci. 2006;78(8):882–7.
Uemura K, Li M, Tsutsumi T, Yamazaki T, Kawada T, Kamiya A, Inagaki M, Sunagawa K, Sugimachi M. Efferent vagal nerve stimulation induces tissue inhibitor of metalloproteinase-1 in myocardial ischemia-reperfusion injury in rabbit. Am J Physiol Heart Circ Physiol. 2007;293(4):H2254–61.
Katare RG, Ando M, Kakinuma Y, Arikawa M, Handa T, Yamasaki F, Sato T. Vagal nerve stimulation prevents reperfusion injury through inhibition of opening of mitochondrial permeability transition pore independent of the bradycardiac effect. J Thorac Cardiovasc Surg. 2009;137(1):223–31.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (No. 81170128). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Andrew Roberts.
Q. Wang and R.-P. Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Q., Li, RP., Xue, FS. et al. Optimal intervention time of vagal stimulation attenuating myocardial ischemia/reperfusion injury in rats. Inflamm. Res. 63, 987–999 (2014). https://doi.org/10.1007/s00011-014-0775-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-014-0775-8