Abstract
Objective and design
Previous studies indicate that endotoxin preconditioning may decrease the inflammatory response and alleviate intestinal mucosal damage caused by sepsis. However, it is not known whether preconditioning with endotoxin might protect the intestinal mucosa after hemorrhagic shock. In this study, we investigated the effect of lipopolysaccharide (LPS) preconditioning on the intestinal mucosa following hemorrhagic shock in a rat model. Given that intestinal toll-like receptor 4 (TLR4) signaling is exaggerated in response to LPS, we further investigated the role of TLR4 signaling in endotoxin tolerance.
Methods
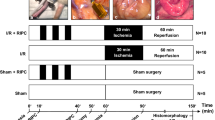
Animals were pre-treated with intra-peritoneal Escherichia coli LPS for 5 days prior to hemorrhagic shock. Animals were bled to achieve a mean arterial pressure (MAP) of 35–40 mmHg, then resuscitated with Ringer solution and the heparinized shed blood to maintain MAP between 90 and 100 mmHg. The distal ileum was harvested after resuscitation and graded for mucosal damage. TNF-α, TLR4, cleaved caspase-3, and intestinal trefoil factor 3 (TFF3) levels were measured at different time points.
Results
Pretreatment with LPS significantly reduced intestinal mucosal damage and protein levels of cleaved caspase-3. Furthermore, animals pre-treated with LPS experienced reduction of TNF-α and increased mucosal expression of TFF3. LPS tolerance was associated with reduced TLR4 expression.
Conclusions
Endotoxin preconditioning can lessen the effects of ischemia and reperfusion injury in intestinal mucosa of a rat model with hemorrhagic shock. It is hypothesized that this effect is mediated via inhibition of TLR4 over-expression.





Similar content being viewed by others
References
Souza DG, Bertini R, Vieira AT, Cunha FQ, Poole S, Allegretti M, et al. Repertaxin, a novel inhibitor of rat CXCR2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br J Pharmacol. 2004;143:132–42.
Eror AT, Stojadinovic A, Starnes BW, Makrides SC, Tsokos GC, Shea-Donohue T. Antiinflammatory effects of soluble complement receptor type 1 promote rapid recovery of ischemia/reperfusion injury in rat small intestine. Clin Immunol. 1999;90:266–75.
Proctor LM, Arumugam TV, Shiels I, Reid RC, Fairlie DP, Taylor SM. Comparative anti-inflammatory activities of antagonists to C3a and C5a receptors in a rat model of intestinal ischaemia/reperfusion injury. Br J Pharmacol. 2004;142:756–64.
Hsieh YH, McCartney K, Moore TA, Thundyil J, Gelderblom M, Manzanero S, et al. Intestinal ischemia-reperfusion injury leads to inflammatory changes in the brain. Shock. 2011;36:424–30.
Sansonetti PJ. The innate signaling of dangers and the dangers of innate signaling. Nat Immunol. 2006;7:1237–42.
Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801.
Chang JX, Chen S, Ma LP, Jiang LY, Chen JW, Chang RM, et al. Functional and morphological changes of the gut barrier during the restitution process after hemorrhagic shock. World J Gastroenterol. 2005;11:5485–91.
Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476–9.
Broad A, Jones DE, Kirby JA. Toll-like receptor (TLR) response tolerance: a key physiological “damage limitation” effect and an important potential opportunity for therapy. Curr Med Chem. 2006;13:2487–502.
Johnson BJ, Le TT, Dobbin CA, Banovic T, Howard CB, Flores FM, et al. Heat shock protein 10 inhibits lipopolysaccharide-induced inflammatory mediator production. J Biol Chem. 2005;280:4037–47.
Ogawa H, Rafiee P, Heidemann J, Fisher PJ, Johnson NA, Otterson MF, et al. Mechanisms of endotoxin tolerance in human intestinal microvascular endothelial cells. J Immunol. 2003;170:5956–64.
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41.
Nemazee D, Gavin A, Hoebe K, Beutler B. Immunology: toll-like receptors and antibody responses. Nature. 2006;441:E4 (discussion E4).
Michelsen KS, Arditi M. Toll-like receptors and innate immunity in gut homeostasis and pathology. Curr Opin Hematol. 2007;14:48–54.
Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–72.
Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–65.
Strieter RM, Kunkel SL, Bone RC. Role of tumor necrosis factor-alpha in disease states and inflammation. Crit Care Med. 1993;21:S447–63.
Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104.
Barrera GJ, Sanchez G, Gonzalez JE. Trefoil factor 3 isolated from human breast milk downregulates cytokines (IL8 and IL6) and promotes human beta defensin (hBD2 and hBD4) expression in intestinal epithelial cells HT-29. Bosn J Basic Med Sci. 2012;12:256–64.
Science TMo, China TotPsRo. Guidance suggestions for the care and use of laboratory animals: MSTPRC Beijing, 2006-9-30.
Endo Y, Shibazaki M, Yamaguchi K, Kai K, Sugawara S, Takada H, et al. Enhancement by galactosamine of lipopolysaccharide(LPS)-induced tumour necrosis factor production and lethality: its suppression by LPS pretreatment. Br J Pharmacol. 1999;128:5–12.
Ypsilantis P, Lambropoulou M, Tentes I, Kortsaris A, Papadopoulos N, Simopoulos C. Mesna protects intestinal mucosa from ischemia/reperfusion injury. J Surg Res. 2006;134:278–84.
Zhai Z, Gomez-Mejiba SE, Gimenez MS, Deterding LJ, Tomer KB, Mason RP, et al. Free radical-operated proteotoxic stress in macrophages primed with lipopolysaccharide. Free Radic Biol Med. 2012;53:172–81.
Murphey ED, Fang G, Varma TK, Sherwood ER. Improved bacterial clearance and decreased mortality can be induced by LPS tolerance and is not dependent upon IFN-gamma. Shock. 2007;27:289–95.
West MA, Heagy W. Endotoxin tolerance: a review. Crit Care Med. 2002;30:S64–73.
Granowitz EV, Porat R, Mier JW, Orencole SF, Kaplanski G, Lynch EA, et al. Intravenous endotoxin suppresses the cytokine response of peripheral blood mononuclear cells of healthy humans. J Immunol. 1993;151:1637–45.
Echtenacher B, Mannel DN. Requirement of TNF and TNF receptor type 2 for LPS-induced protection from lethal septic peritonitis. J Endotoxin Res. 2002;8:365–9.
Schwarz NT, Engel B, Eskandari MK, Kalff JC, Grandis JR, Bauer AJ. Lipopolysaccharide preconditioning and cross-tolerance: the induction of protective mechanisms for rat intestinal ileus. Gastroenterology. 2002;123:586–98.
Tasaki K, Ruetzler CA, Ohtsuki T, Martin D, Nawashiro H, Hallenbeck JM. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res. 1997;748:267–70.
Wong WM, Poulsom R, Wright NA. Trefoil peptides. Gut. 1999;44:890–5.
Rupani B, Caputo FJ, Watkins AC, Vega D, Magnotti LJ, Lu Q, et al. Relationship between disruption of the unstirred mucus layer and intestinal restitution in loss of gut barrier function after trauma hemorrhagic shock. Surgery. 2007;141:481–9.
Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179:4808–20.
Watanabe T, Higuchi K, Kobata A, Nishio H, Tanigawa T, Shiba M, et al. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut. 2008;57:181–7.
Wang J, Ouyang Y, Guner Y, Ford HR, Grishin AV. Ubiquitin-editing enzyme A20 promotes tolerance to lipopolysaccharide in enterocytes. J Immunol. 2009;183:1384–92.
Cario E, Brown D, McKee M, Lynch-Devaney K, Gerken G, Podolsky DK. Commensal-associated molecular patterns induce selective toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am J Pathol. 2002;160:165–73.
Furrie E, Macfarlane S, Thomson G, Macfarlane GT. Toll-like receptors-2, -3 and -4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology. 2005;115:565–74.
Acknowledgments
The project was supported by National Natural Science Foundation of China (81071761) and Guangdong National Natural Science Foundation (10151008901000135).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
R. Chang and Y. Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chang, R., Wang, Y., Chang, J. et al. LPS preconditioning ameliorates intestinal injury in a rat model of hemorrhagic shock. Inflamm. Res. 63, 675–682 (2014). https://doi.org/10.1007/s00011-014-0740-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-014-0740-6