Abstract
Objective and design
To determine alternate therapeutic measures to combat Staphylococcus aureus induced arthritis. Thus, azithromycin was combined with riboflavin, which may combat the ROS production and inflammation.
Methods
An in vivo model of S. aureus infection-induced arthritis was set up by infecting mice with 5 × 106 bacterial cell/mouse. S. aureus was administered intravenously. Azithromycin and riboflavin was injected intraperitoneally at a single dose of 100 and 20 mg/kg body, respectively. The mice were sacrificed at 3, 9, 15 days post infection (dpi). TNF-α, IFN-γ, IL-6 and IL-10 from serum and SOD, catalase and reduced glutathione concentration were observed in hepatic, cardiac, renal and splenic tissue.
Results
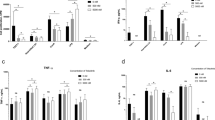
CFU was found very prominent in spleen and joints and reduced in blood at 3 and 9 dpi. However, treatment with azithromycin and riboflavin completely eradicated the bacteria from blood and spleen. TNF-α, IFN-γ, IL-6, and MCP-1 were induced due to infection which were downregulated by treatment with azithromycin and riboflavin. Infected mice were also found to have altered antioxidant status, measured in terms of reduced glutathione and anti-oxidant enzymes such as SOD and catalase.
Conclusion
These changes were found to be ameliorated when the animals were co-treated with azithromycin and riboflavin.







Similar content being viewed by others
References
Anwar S, Prince LR, Foster SJ, Whyte MK, Sabroe I. The rise and rise of Staphylococcus aureus: laughing in the face of granulocytes. Clin Exp Immunol. 2009;157:216–24.
Canvin JM, Goutcher SC, Hagig M, Gemmell CG, Sturrock RD. Persistence of Staphylococcus aureus as detected by polymerase chain reaction in the synovial fluid of a patient with septic arthritis. Br J Rheumatol. 1997;36:203–6.
Mathews CJ, Weston VC, Jones A, Field M, Coakley G. Bacterial septic arthritis in adults. Lancet. 2010;375:846–55.
Abdelnour A, Bremell T, Holmdahl R, Tarkowski A. Role of T lymphocytes in experimental Staphylococcus aureus arthritis. Scand J Immunol. 1994;39:403–8.
Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3:948–58.
Darley ES, MacGowan AP. Antibiotic treatment of gram-positive bone and joint infections. J Antimicrob Chemother. 2004;53:928–35.
Nair SP, Williams RJ, Henderson B. Advances in our understanding of the bone and joint pathology caused by Staphylococcus aureus infection. Rheumatology (Oxford). 2000;39:821–34.
Liu GY. Molecular pathogenesis of Staphylococcus aureus infection. Pediatr Res. 2009;65:71R–7R.
Sakiniene E, Bremell T, Tarkowski A. Addition of corticosteroids to antibiotic treatment ameliorates the course of experimental Staphylococcus aureus arthritis. Arthritis Rheum. 1996;39:1596–605.
Nizet V. Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. J Allergy Clin Immunol. 2007;120:13–22.
Dormandy TL. Free-radical pathology and medicine. A review. J R Coll Physicians Lond. 1989;23:221–7.
Mirshafiey A, Mohsenzadegan M. The role of reactive oxygen species in immunopathogenesis of rheumatoid arthritis. Iran J Allergy Asthma Immunol. 2008;7:195–202.
Bodamyali T, Stevens CR, Billingham ME, Ohta S, Blake DR. Influence of hypoxia in inflammatory synovitis. Ann Rheum Dis. 1998;57:703–10.
Girard AE, Girard D, English AR, Gootz TD, Cimochowski CR, Faiella JA, Haskell SL, Retsema JA. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987;31:1948–54.
Girard AE, Girard D, Retsema JA. Correlation of the extravascular pharmacokioetics of azithromycin with in vivo efficacy in models of localized infection. J Antimicrob Chemother. 1990;25:61–71.
Meyer AP, Bril-Bazuin C, Matfie H, Van Den Broek PJ. Uptake of azithromycin by human monocytes and enhanced intracellular antibacterial activity against Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:2318–22.
Cheung AL, Bayer AS, Zhang G, Gresham H, Xiong YQ. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol Med Microbiol. 2004;40:1–9.
Mazur-Bialy AI, Majka A, Wojtas L, Kolaczkowska E, Plytycz B. Strain-specific effects of riboflavin supplementation on zymosan-induced peritonitis in C57BL/6 J, BALB/c and CBA mice. Life Sci. 2011;88:265–71.
Martins SA, Combs JC, Noguera G, Camacho W, Wittmann P, Walther R, et al. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: a potential new treatment for infectious keratitis. Invest Ophthalmol Vis Sci. 2008;49:3402–8.
Mal P, Ghosh D, Bandyopadhyay D, Bishayi B. Ampicillin alone and in combination with riboflavin modulates Staphylococcus aureus infection induced septic arthritis in mice. Indian J Exp Biol. 2012. In press.
Majumdar S, Dutta K, Manna SK, Basu A, Bishayi B. Possible protective role of chloramphenicol in TSST-1 and coagulase-positive Staphylococcus aureus-induced septic arthritis with altered levels of inflammatory mediators. Inflammation. 2011;34:269–82.
Sen R, Das D, Bishayi B. Staphylococcal catalase regulates its virulence and induces arthritis in catalase deficient mice. Indian J Physiol Pharmacol. 2009;53:307–17.
Yao L, Berman JW, Factor SM, Lowy FD. Correlation of histopathologic and bacteriologic changes with cytokine expression in an experimental murine model of bacteremic Staphylococcus aureus infection. Infect Immun. 1997;65:3889–95.
Tissi L, von Hunolstein C, Mosci P, Campanelli C, Bistoni F, Orefici G. In vivo efficacy of azithromycin in treatment of systemic infection and septic arthritis induced by type IV group B Streptococcus strains in mice: comparative study with erythromycin and penicillin G. Antimicrob Agents Chemother. 1995;39:1938–47.
Verdrengh M, Tarkowski A. Riboflavin in innate and acquired immune responses. Inflamm Res. 2005;54:390–3.
Burchill MA, Nardelli DT, England DM, DeCoster DJ, Christopherson JA, Callister SM, et al. Inhibition of interleukin-17 prevents the development of arthritis in vaccinated mice challenged with Borrelia burgdorferi. Infect Immun. 2003;71:3437–42.
Dixon RA. Curative effects of tobramycin or gentamicin therapy on mouse arthritis caused by Mycoplasma pulmonis. Antimicrob Agents Chemother. 1981;20:321–6.
Chattopadhyay A, Biswas S, Bandyopadhyay D, Sarkar C, Datta AG. Effect of isoproterenol on lipid peroxidation and antioxidant enzymes of myocardial tissue of mice and protection by quinidine. Mol Cell Biochem. 2003;245:43–9.
Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellmanʼs reagent. Anal Biochem. 1968;25:192–205.
Martin JP, Dailey M, Sugarman E. Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch Biochem Biophys. 1987;255:329–36.
Beers RF Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–40.
Dutta K, Mishra MK, Nazmi A, Kumawat KL, Basu A. Minocycline differentially modulates macrophage mediated peripheral immune response following Japanese encephalitis virus infection. Immunobiology. 2010;215:884–93.
Johnson WM, Tyler SD, Ewan EP, Ashton FE, Pollard DR, Rozee KR. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J Clin Microbiol. 1991;29:426–30.
Becker K, Roth R, Peters G. Rapid and specific detection of toxigenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for amplification and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes, and toxic shock syndrome toxin 1 gene. J Clin Microbiol. 1998;36:2548–53.
Das D, Das A. Analysis of variance. In: Das D, editor. Statistics in biology and psychology. Calcutta: Academic; 2005. pp. 280–93.
Bremell T, Lange S, Yacoub A, Ryden C, Tarkowski A. Experimental Staphylococcus aureus arthritis in mice. Infect Immun. 1991;59:2615–23.
Sasaki H, Hou L, Belani A, Wang CY, Uchiyama T, Muller R, et al. IL-10, but not IL-4, suppresses infection-stimulated bone resorption in vivo. J Immunol. 2000;165:3626–30.
Yoshii T, Magara S, Miyai D, Nishimura H, Kuroki E, Furudoi S, et al. Local levels of interleukin-1beta, -4, -6 and tumor necrosis factor alpha in an experimental model of murine osteomyelitis due to staphylococcus aureus. Cytokine. 2002;19:59–65.
Moore KW, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90.
Hultgren OH, Stenson M, Tarkowski A. Role of IL-12 in Staphylococcus aureus-triggered arthritis and sepsis. Arthritis Res. 2001;3:41–7.
Mal P, Dutta S, Bandyopadhyay D, Dutta K, Basu A, Bishayi B. Gentamicin in combination with ascorbic acid regulates the severity of Staphylococcus aureus infection induced septic arthritis in mice. Scand J Immunol. 2012. In press.
Acknowledgments
The corresponding author (Biswadev Bishayi) thanks the Council of Scientific and Industrial Research (CSIR) Ministry of Human Resource Development, Government of India, New Delhi, India for funding this project. The author (BB) is indebted to Dr. Sunil Kumar Manna, Scientist and Head, Immunology Division, Centre for DNA Fingerprinting and Diagnostics, Hyderabad, India for providing us with the primers for TSST-1 and coagulase. KD is recipient of Research Associateship in Biotechnology and Life Sciences from Department of Biotechnology, Govt. of India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Mal, P., Dutta, K., Bandyopadhyay, D. et al. Azithromycin in combination with riboflavin decreases the severity of Staphylococcus aureus infection induced septic arthritis by modulating the production of free radicals and endogenous cytokines. Inflamm. Res. 62, 259–273 (2013). https://doi.org/10.1007/s00011-012-0574-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-012-0574-z