Abstract
Sepsis is the most frequent cause of death in noncoronary intensive care units. In the past 10 years, progress has been made in the early identification of septic patients and their treatment. These improvements in support and therapy mean that mortality is gradually decreasing, however, the rate of death from sepsis remains unacceptably high. Immunotherapy is not currently part of the routine treatment of sepsis. Despite experimental successes, the administration of agents to block the effect of sepsis mediators failed to show evidence for improved outcome in a multitude of clinical trials. The following survey summarizes the current knowledge and results of clinical trials on the immunotherapy of sepsis and describes the limitations of our knowledge of the pathogenesis of sepsis. Administration of immunomodulatory drugs should be linked to the current immune status assessed by both clinical and molecular patterns. Thus, a careful daily review of the patient’s immune status needs to be introduced into routine clinical practice giving the opportunity for effective and tailored use of immunomodulatory therapy.
Similar content being viewed by others
Introduction
Sepsis is the most frequent cause of death in noncoronary intensive care units (ICUs). It is a serious disease with a high mortality and represents an immense financial burden on the health care system. In the past two decades, the incidence of sepsis has been on the rise not only in developing countries but also in the USA and countries of Western Europe (Martin 2012). In developed countries, the incidence of sepsis is 2 % of all hospitalizations and 6–30 % of ICU patients; even more dramatic is the situation in developing countries (Jawad et al. 2012). The problem is the high mortality of severe sepsis, which is currently higher than that of myocardial infarction; even worse outcomes are revealed when patients with septic shock are considered (Esper and Martin 2007).
In the past decades, progress has been made in the education, prevention, identification, treatment and rehabilitation of septic patients. All of these important advancements together have gradually ameliorated the individual burden of sepsis; nevertheless, it remains unacceptably high. Moreover, due to the ever growing incidence of sepsis, the overall number of patients who die from sepsis continues to increase (Adhikari et al. 2010). Martin et al. (2009) analyzed more than 11,000 patients included in an international registry comprising severe sepsis cases. In that retrospective study, 57 % of patients suffered from Gram-negative, 44 % from Gram-positive, and 11 % from mycotic infections. The lungs were the primary source of infection in 47 % of patients, abdominal infection was found in 23 %, and urinary tract infection in 8 %. The total mortality reached almost 50 %. The European Sepsis Occurrence in Acutely Ill Patients study revealed that the incidence of sepsis in European ICU’s is 33 % with an overall mortality of 27 % (Vincent et al. 2006). It is not surprising that the scientific community continues to call for improved treatment options, including a timely diagnosis and active surveillance in hospitalized patients at high risk of sepsis development.
Immune Mechanisms of Sepsis
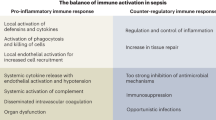
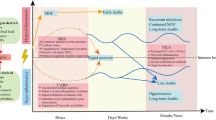
While a specific molecular pattern of sepsis is still missing, it is widely accepted that dysregulation of host response pathways and consecutive defects in homeostasis are critical pathogenic factors in the development of the clinical signs of sepsis (Deutschman and Tracey 2014). The concept of a hyperinflammatory syndrome which has dominated scientific discussion over the past two decades has been challenged and at present, sepsis is seen more as a dynamic syndrome characterized by many, often antagonistic phenomena (Cavaillon et al. 2014). Hence, sepsis should be viewed as a dynamic process spanning hyperinflammatory responses as well as anergy and immunoparalysis. It is increasingly understood that the previous concept of a proinflammatory process followed by a compensatory anti-inflammatory phase does not represent the common clinical picture. Rather, the two processes progress with a significant degree of synchrony, although not necessarily with the same time course; systemic immunosuppression typically dominates (Fig. 1). A unique study highlighting immunosuppression in sepsis patients was published in 2011 (Boomer et al. 2011). Boomer et al. (2011) conducted a functional study designed to assess immune system status in patients who had died due to sepsis. The authors isolated the lung and spleen cells from these patients and compared them with cells from trauma victims. After immunophenotyping, they elucidated immunological status and demonstrated functional immunosuppression in patients dying from sepsis, manifesting itself largely by decreased production of both pro- and anti-inflammatory cytokines, reduced monocyte HLA-DR expression, and a decreased immunocompetent cell count. Interestingly, sepsis-induced immunosuppression could be reversed by removing the cells from the sepsis “milieu”. Clinical findings in these patients include skin test anergy to memory cell-specific antigens, leukopenia, and increased susceptibility to infection (Hotchkiss et al. 2013). Typically, the immune response of sepsis patients is a hyperinflammatory systemic response and/or a state of “immunoparalysis” characterized by low monocyte HLA-DR expression and decreased tumor necrosis factor (TNF)-α production ex vivo after lipopolysaccharide (LPS) stimulation. Characteristic features of the hyperinflammatory phase include increased production of cytokines, i.e., TNF-α, macrophage migration-inhibiting factor, and high-mobility group box 1 protein (Huang et al. 2010). By contrast, the compensatory anti-inflammatory response syndrome, which can yield immunoparalysis, typically involves the production of interleukin (IL)-4, IL-10, IL-11, IL-13, transforming growth factor β, granulocyte and granulocyte-macrophage colony-stimulating factors (G-CSF and GM-CSF, respectively), soluble TNF-α receptors and IL-1 receptor antagonists. Patients with sepsis have features of a Th1-oriented response with the production of proinflammatory cytokines, initially. However, if sepsis persists, a shift towards an anti-inflammatory immunosuppressive state occurs with findings of anergy of effector T cells (loss of costimulatory and MHC class II molecules by antigen-presenting cells, increased apoptosis of T and B cells representing the switch from a protective Th1 to a less protective Th2 profile of immune response; Hotchkiss and Karl 2003; Lederer et al. 1999).
Apart from infection, immunoparalysis can be induced by noninfectious causes, such as trauma, ischemia and burns. A summary of the mechanisms responsible for the status of immunosuppression in sepsis is given in Table 1.
Limitations of Our Current Knowledge
Experimental Sepsis Model
The gold standard used for sepsis research is the peritonitis model of cecal ligation, whereby the gastrointestinal flora is released and translocated into the blood circulation. However, it is obvious that this kind of model does not correspond with the clinical reality in terms of mechanisms, origin and causative pathogens (Dejager et al. 2011; Rittirsch et al. 2007). A recent study revealed that comparing the immune systems of mice and humans is very problematic, to say the least (Seok et al. 2013; Shay et al. 2013). Therefore, it seems important to continue with the development of new sepsis models. The use of a humanized murine sepsis model might be a step towards this goal (Melican and Dumeni 2013). Furthermore, clinical observational studies applying highly similar methods from proteomics and functional genomics might better describe the complex immunological changes from the onset of sepsis until death or discharge in well characterized patient cohorts (Ito et al. 2012).
Sepsis Biomarkers
“The cause of most human diseases lies in the functional dysregulation of protein-interactions” (Liotta et al. 2001). Recent research suggests that every cell in the body leaves a record of its physiological state in the product it sheds to the blood. It is therefore thought that the blood contains a treasure trove of previously unstudied biomarkers that could reflect the ongoing physiological state of all tissues (Liotta et al. 2003). In sepsis, specific protein markers are already known to correlate with the magnitude of sepsis related organ damage. Some protein markers such as procalcitonin have also been proven to be useful for disease prognosis and therapeutic monitoring (Russwurm and Reinhart 2004). To date, there is no “gold standard” diagnostic test for sepsis. We are presenting the most important ones available to clinicians and researchers in short summary.
In recent years, a number of studies have been published comparing the usefulness of determination of C-reactive protein and procalcitonin in differential diagnostics of infectious and noninfectious inflammation. Meta-analyses showed higher specificity and sensitivity of procalcitonin in comparison with C-reactive protein, however, neither procalcitonin nor C-reactive protein fulfill the role of an ideal biomarker in the diagnostics of sepsis (Simon et al. 2004; Tang et al. 2007).
CD64 is a high affinity receptor for IgG (FcγRI) and represents marker of neutrophil activation. It is constitutively expressed by macrophages and monocytes only, whereas its expression on neutrophils occurs after activation by cytokines, i.e., interferon (IFN)-γ and G-CSF. A number of studies have been published on the importance of CD64 for diagnostics of sepsis. The Bhandari study demonstrated sensitivity of CD64 at 80 % and specificity of 79 % with neonatal sepsis (Bhandari et al. 2008). In adults, Icardi et al. (2009) showed a very good predictive value of CD64 for clinical and microbiological diagnosis of sepsis with sensitivity of 94.6 % and specificity of 88.7 %. Similar results were confirmed in other studies (Elawady et al. 2014; Nuutila 2010). The measurements of neutrophil CD64 expression thus represent a positive step in the differential diagnosis of systemic inflammatory response syndrome (SIRS) of an infectious vs noninfectious etiology.
In 2005, a new biomarker sCD14-ST was discovered and named as presepsin (Yaegashi et al. 2005). CD14 is a glycoprotein on membrane surfaces of monocytes/macrophages, and acts as a receptor for complexes of lipopolysaccharide (LPS) and LPS-binding protein (LBP). CD14 activates the Toll-like receptor (TLR)4 proinflammatory cascade in the presence of infectious agents. Two forms of CD14 are present membrane CD14 and soluble CD14 (sCD14). Complex LPS-LBP-CD14 is shed into the circulation and plasma protease generates sCD14 molecule called sCD14 subtype (sCD14-ST)-presepsin. Recently, clinical studies were published on the relationship between presepsin and sepsis. Presepsin levels are increased in septic patients with no significant difference between patients with Gram-positive or Gram-negative infection. Presepsin appears to be very early biomarker of sepsis with place in the clinical diagnostics and may be of interest for future studies (Carpio et al. 2015; Zhang et al. 2015).
TREM-1 is a cell surface receptor expressed on the myeloid cells, it belongs to immunoglobulin superfamily. In addition to mTREM-1, which is expressed on monocytes, sTREM-1 can be detected in the serum or bronchoalveolar lavage. In recent years, TREM-1 has been studied as a factor mediating the inflammatory response of the body to infection. The determination of the levels of its soluble form (sTREM-1) or determination of expression of TREM-1 on monocytes (mTREM-1) has been investigated as a perspective diagnostic method to distinguish infectious from noninfectious etiology of the inflammation (Adib-Conquy et al. 2007; Marioli et al. 2014). At the moment, TREM-1 has not been adopted formally in the diagnosis or tracking of sepsis but it is worth watching for future developments.
Sepsis as a Mediator’s Disease
Sepsis is considered to be “a mediator’s disease”. It has been our understanding that we can reverse the course of sepsis by blocking these mediators. The devastating effect of TNF-α in sepsis has been largely confirmed. However, TNF-α also has a positive effect during sepsis in the host. The protective effects of endogenous TNF were unambiguously confirmed by studies carried out by Echtenacher. Stated simply, the presence of endogenous TNF and its receptors is a prerequisite of the successful outcome of sepsis (Echtenacher et al. 2003). The situation is similar for IL-6. The essential role of IL-6 as a cytokine to control local and general inflammatory responses in sepsis has been confirmed (Xing et al. 1998). Its role is irreplaceable and irretrievable in this context. On the other hand, IL-10 is considered to be the main anti-inflammatory immunosuppressive cytokine with an irreplaceable role in preventing the development of multiple organ failure in experimental animals (Sewnath et al. 2001).
The Role of the Immune System in Sepsis
Under physiological conditions, the most important role of the immune system is to maintain homeostasis. In sepsis, there is a significant homeostatic imbalance.
There are two theories which attempt to explain the role of the immune system in the pathogenesis of sepsis: (1) immunosuppression that is present in sepsis has its purpose, which is to eliminate the significant proinflammatory activity initiated by the infectious agent; (2) immunosuppression that accompanies sepsis is a pathological finding caused by the interaction of macroorganism and infectious agent.
In this context, it is necessary to recall the phenomenon of “disease tolerance” as one of the body’s protective strategies. This phenomenon has been clearly demonstrated and it helps the body to better fight the present infection. At the same time, the tolerance of the host’s main systems varies. This tolerance is related to a debilitating infectious agent, as well as to the host injury caused by its own immune system (Medzhitov et al. 2012). The injury caused by one’s own immune system may be compared to an autoaggressive inflammatory reaction. The phenomenon of endotoxin tolerance may serve as an example of tolerance in sepsis. A recently published work by Spanish authors confirmed the rating of endotoxin tolerance as a significant immunosuppressive mechanism in septic patients and demonstrated their findings on the level of the host genome (Pena et al. 2014). Mitochondrial dysfunction found in septic patients is a widely discussed issue that has been confirmed (Singer 2014). This represents another avenue of immune system dysfunction in sepsis. Both innate and adaptive immunity are closely linked to mitochondrial function, and its malfunction is one of the most important findings in serious primary immunodeficiency (Walker et al. 2014). Treatment for mitochondrial dysfunction in septic patients represents the promising tool for the future (Corrêa et al. 2015; Morel and Singer 2014; Zheng et al. 2015).
Immunotherapy of Sepsis
We can divide immunotherapy of sepsis into the following: (a) treatments combining substitution and immune-modulating effects, represented by intravenous immunoglobulins (IVIG), growth factors and corticosteroids and (b) inhibitory treatments—using substances to block the effect of mediators or signaling molecules.
The Beginnings of Immunomodulation Therapy
The major role played by endotoxin in the pathogenesis of sepsis led to the use of therapies to block its activity as early as the 1970s and 1980s. This treatment was pioneered by A. Braude and E. Ziegler, who immunized volunteers with E. coli J5 bacteria and then applied their immune plasma to patients with G-sepsis. Initial results were encouraging (Ziegler et al. 1991) but later studies failed to confirm their conclusions (McCloskey et al. 1994). The development of technologies enabling the production of monoclonal antibodies led to another study. The use of the monoclonal antibodies—HA1A, E5 against one of the basic structural components of endotoxin, lipid A—produced similar results (Derkx et al. 1999).
Intravenous Immunoglobulins
Intravenous immunoglobulin treatment of patients with sepsis represents one of the controversial aspects of the existing immunomodulatory therapy for sepsis. What is the rational basis for indicating this treatment? The effects of IVIG are the following: (a) substitution and inactivation—neutralization of endotoxins and exotoxins, increased clearance of endotoxins, reduced adherence of bacteria, migration and invasiveness; (b) immunomodulation—stimulation of leukocytes and germicides, increased oxidative inflammation when using 7S-IVIG or intact IgG, reduction of endotoxins by induced oxidative inflammation when using 5S-IVIG or a Fab IgG fragment and IgM, increased serum opsonic activity; (c) immunomodulation effect on cytokine production—reduced production of proinflammatory mediators, increased production of anti-inflammatory mediators, neutralization of cytokines using anticytokine antibodies.
Two meta-analyses on the administration of IVIG to patients with sepsis were recently presented (Kreymann et al. 2007; Turgeon et al. 2007), all of them in favor of the administration of IVIG. However, a critical analysis of these published studies highlights their methodological shortcomings. The last meta-analysis showed that polyclonal IVIG reduced mortality among adults with sepsis but this benefit was not seen in trials with low risk of bias (Alejandria et al. 2013). One of the most recent major studies was a multicentric study, (score-based IgG therapy of patients with sepsis), which failed to demonstrate any positive effect of the administration of IVIG on mortality levels at doses of 0.9 g/kg body weight (Werdan et al. 2007). The results of studies of IVIG administration in neonates with sepsis have long supported the positive effect of this approach on mortality (Ohlsson and Lacy 2004). However, the results of a multicentric study published in 2012 did not support these findings (INIS Collaborative Group et al. 2011).
There are still centers where IVIG is routinely administered. An Italian study shows the benefit of administering IVIG containing IgM in the early stages of severe sepsis and septic shock (Berlot et al. 2012), while the Rankin study defends the beneficial effect of IVIG in patients having cardiac surgery (Rankin et al. 2011). Both of these studies focus more on the immunomodulatory effect of IVIG, not the substitution effect.
Streptococcal and staphylococcal septic shock and necrotising staphylococcal lung infections with evidence of Panton-Valentine leucocidin present a special group of infectious diseases or sepsis. For these patients, the administration of 1–2 g/kg body weight over a period of 3 days is recommended. It should be pointed out that experiments have shown that polyclonal immunoglobulins are less effective at inhibiting the staphylococcal superantigen compared with the streptococcal one. It is therefore recommended to treat cases of staphylococcal septic shock with higher doses. Although no major randomized study exists, individual case studies have demonstrated the effectiveness of IVIG treatment (Raithatha and Bryden 2012). The administration of IVIG is not currently recommended for the treatment of sepsis and septic shock in adults, and the possible presence of hypogammaglobulinemia in patients with sepsis is not taken into consideration. The treatment of sepsis in patients with neutropenia with IVIG did not show a significant difference in survival in a randomized controlled trial (Hentrich et al. 2006). 2014 updated guidelines from the Infectious Diseases Working Party of the German Society of Hematology and Medical Oncology states: there is moderate degree of evidence to support the use of IVIG in sepsis (Penack et al. 2014).
The current strategy is to test and to use IVIG in more homogenous cohorts of patients. One example is the study on IVIG administration for patients with community acquired pneumonia, which is being prepared (Welte et al. 2015).
Treatment with Cytokine Inhibitors
The role of cytokines in the pathogenesis of sepsis is clear. On the one hand, cytokines are an essential part of the organism’s adequate reaction to an infectious insult. On the other hand, the excessive production of cytokines, which is disproportionate to the size of the primary insult, is a source of damage to organs and tissues, manifesting itself in various degrees of organ dysfunction. On the basis of preclinical studies, cytokines involved in organ damage have been identified: TNF-α and IL-1. TNF-α is a key mediator of LPS-induced sepsis. The administration of monoclonal antibodies against TNF-α or the receptor for TNF prevented the development of symptoms of sepsis in an experimental model (Tracey et al. 1987). A whole range of clinical studies were subsequently carried out, but with no demonstrable clinical benefit (Arndt and Abraham 2001). An interesting evaluation of experimental studies using TNF inhibitors was made by Lorente and Marshall (2005). During a detailed analysis, they found that the effectiveness of a treatment differed depending on the model of sepsis used. The most positive effect was present in a model involving the application of endotoxin, where bacterial growth was prevented, while in contrast, the injurious effect of treatment was shown in the model with intracellular pathogens. Better efficacy was shown in a bacterial model, compared with the one of localized infection (pneumonia) and the importance of the time of application was clear: prophylactic administration produced a better effect than the model in which administration followed the infectious insult.
A similar situation arose in studies involving the use of an IL-1 inhibitor or antagonists of the IL-1 receptor. IL-1 exists in two different forms: IL-1α and IL-1β, with IL-1β being dominant in plasma. IL-1 acts in synergy with TNF in sepsis and contributes to organ and cell damage. Although the primary clinical study did demonstrate a beneficial effect on mortality, subsequent studies have not confirmed this effect (Fisher et al. 1994; Opal et al. 1997). No further clinical studies are currently in progress.
Another example of immunomodulatory therapy is related to IL-7 and IL-15. IL-7 induces T lymphocytes proliferation and through this effect is able to restore the delayed type hypersensitivity response, which is decreased in sepsis (Buckley 2004; Unsinger et al. 2010). In preclinical studies, IL-7 administration increased the number of CD4+ and CD8+ T cells in peripheral blood, but had no effect on the number of regulatory T cells. T cells are typically depleted in septic patients through apoptosis; therefore, the potential therapeutic benefit is obvious. IL-15 plays important role in maturing and activation of the T cells, NK cells and NKT cells. In experimental model, IL-15 was able to reduce apoptosis of immunocompetent cells and to decrease mortality (Inoue et al. 2010).
The Use of Inhibitors of TLR Pathways
The discovery of innate immunity signaling mechanisms has provided more options for therapeutic intervention. Both immunocompetent and immunocompromised cells have on their surfaces the so-called PRRs (pattern recognition receptors), which are able to distinguish between the bacterial patterns of individual infectious agents (pathogen associated molecular patterns). One of the most important receptor groups is TLRs. TLRs are transmembrane receptors that recognize a range of molecules: LPS, peptidoglycan, bacterial lipoproteins and lipopeptides and lipoteichoic acid. The activation of these receptors through nuclear factor (NF)-κB and activator protein (AP)-1 results in the expression of genes associated with the inflammatory response. TLR4 is responsible for recognizing LPSs, which as has already been mentioned, are one of the main mediators of sepsis. During the onset of infection, after the initial phase of TLR4 activation on immunocompromised cells, a secondary response occurs with the activation of endothelial cells and the production of adhesive molecules, macrophage infiltration and increased vascular permeability. The result is the start of a coagulation cascade, impaired tissue perfusion and organ failure. One of the ways to prevent the activation of the inflammatory response process with its devastating effects, is to block the activation of TLR. A number of substances that block the interaction of TLR with LPS exist, with the working titles CRX-526, E5531, E5564 and TAK-242, but only two of them have been used in clinical trials. In 2012, a clinical study on the substance E5564 (Eritoran) which blocked the MD2-TLR4 complex as well as on TAK-242 in both cases with negative results, was suspended. In the case of the study on MD2-TLR4 blocking (using the Eritoran preparation) findings of the potential adverse consequences of this therapy for patients with Gram-positive sepsis are important (Opal et al. 2013).
Corticosteroids
The mechanism for the action of corticosteroids is complex. Corticosteroids bind to corticosteroid receptors, with which they form an active corticosteroid-corticosteroid receptor complex (CS-CSR). The CS-CSR complex can bind directly to proinflammatory transcription factors, such as AP-1 or NF-κB thereby preventing the function of these transcription factors and their subsequent adverse effects. We are talking about the indirect genomic effects of corticosteroids (De Bosscher et al. 2003). The direct genome effect is associated with the penetration of the CS-CSR complex into the nucleus, where it binds to nuclear DNA sequences known as GRE (glucocorticoid response elements). The result of this interaction is the formation of activator or repressor proteins, which cause the activation or inhibition of the transcription process (Hebbar and Archer 2003). The mechanism behind nongenomic actions is mediated by the interaction between the corticosteroid receptor associated with the membrane and the so-called “second messenger” (Hafezi-Moghadam et al. 2002). The rationale for using corticosteroids in sepsis is based on the evidence of critical illness related corticosteroid insufficiency, first described by Marik et al. (2008). Signature advances were made in the 1970s and 1980s through work demonstrating adverse effect of high doses of corticosteroids on the mortality of patients with septic shock (Cronin et al. 1995). A breakthrough occurred with the work of Annane, which showed the beneficial effects of substitute doses of corticosteroids on patients with early onset severe septic shock and a demonstrated deficiency in the production of endogenous corticosteroids (Annane et al. 2002). The conclusions of the Annane study were not confirmed by the Corticus study, which showed more adverse side effects resulting from their administration (Sprung et al. 2008). Even today, the available data do not allow us to clearly articulate the role played by steroids in the treatment of sepsis or septic shock and the prevailing consensus is that there is a need for additional controlled studies. The administration of substitution doses of corticosteroids of 200 mg/day for patients with septic shock who do not react to adequate fluid resuscitation and the administration of vasopressors is currently recommended—Grade 2C (Dellinger et al. 2013).
Growth Factors
Studies carried out over recent years, which have clearly shown the presence of immunosuppression effects in patients with sepsis, have focused attention on the use of immunostimulatory treatments. G-CSF, GM-CSF and IFN-γ are used for immunostimulatory therapy. G-CSF was primarily used for patients with neutropenia, immunosuppression and a high risk of infection after a stem cell transplant or antitumor therapy. G-CSF has an important anti-inflammatory effect and enhances bacterial clearance. For these reasons, it is used on patients with sepsis, but clinical studies have not confirmed any effect on mortality (Nelson et al. 1998; Root et al. 2003). The studies indicate that the effect of G-CSF could relate in different ways to the site of the infection and the etiological agent. While G-CSF had a positive outcome on extravascular sepsis with a localized focus of infection, in the case of sepsis-induced by intravascular administration of the infection agent, it had no effect (Sevransky et al. 2004). A more fundamental difference was found with regard to the type of infectious agent (Karzai et al. 1999). GM-CSF stimulated the production and function of neutrophils and monocyte granulocytes. The results of clinical studies are inconsistent. Studies by US authors have not demonstrated the benefits of this treatment on the mortality of patients with severe sepsis, but an effect was observed on the functional parameters of the immune system, with an increase in the expression of adhesive molecules and an increased expression of HLA-DR on monocytes (Presneill et al. 2002; Rosenbloom et al. 2005). A study of the administration of GM-CSF to surgical patients with nontraumatic abdominal sepsis revealed the following effects: reduced length of stay in the hospital, fewer infectious complications and lower costs (Orozco et al. 2006). A recent multicentre study has shown that GM-CSF does contribute to improved immune system function (Schefold 2011). Similar results have been obtained following the administration of GM-CSF to neonates and infants. A study by Bilgin et al. (2001) demonstrated the effect of GM-CSF administration on the mortality of neutropenic children, although a recent meta-analysis and PROGRAMS trial have not confirmed this (Carr et al. 2003, 2009). IFN-γ exhibits a wide range of antibacterial effects by impacting the function of the immune system: it increases the expression of HLA-DR molecules and the Fc receptor on monocytes, supports the function of NK cells and participates in monocyte activation by macrophages in terms of their bactericidal activity (Matsumura et al. 1990). Clinical studies have demonstrated the beneficial effect of IFN-γ on these functions, without any reduction in mortality (Döcke et al. 1997; Leentjens et al. 2012; Nakos et al. 2002).
Experimental Approaches to Immunomodulatory Treatment
In addition to the types of immunomodulatory treatment outlined above, where clinical studies have been concluded or are currently underway, other approaches are being tested experimentally. One of these is the blocking of the so-called negative costimulatory molecules.
Inhibitors of Negative Costimulatory Molecules
Negative costimulatory molecules are important mediators of the development of humoral and cellular immunity. An example of a negative costimulatory molecule is the PD-1 (programmed cell death) protein. It is expressed on activated CD4+ and CD8+ lymphocytes. It inhibits the proliferation of T lymphocytes and reduces the production of inflammatory cytokines. Experiments have shown that in the absence of this protein, animals have enhanced bacterial clearance and reduced mortality due to sepsis. The administration of anti-PD-1 antibodies prevented apoptosis of immunocompetent cells with induced sepsis and improved survival in a model of intra-abdominal sepsis (Brahmamdam et al. 2010). Other examples of experimental treatments are listed in Table 2.
Current and Future Strategies of Immunotherapy in Sepsis
This overview shows that none of the above-mentioned strategies has led to a significant change in mortality and clinical outcome. In 2014, a study from Australia and New Zealand was published documenting a reduction in the mortality of patients with severe sepsis and septic shock 35–18.4 % between 2000 and 2012 without the use of any immunomodulation therapy (Kaukonen et al. 2014). In the light of this data, we have to ask whether there is a real chance of achieving a further improvement in clinical outcome by administering appropriate immunotherapy?
Immune System Monitoring—When a Little Can Mean a Lot
There is no doubt that sepsis leads to a significant dysfunction of the immune system; however, the evaluation of immune dysfunction is not a common clinical practice. There are few clinical sites where immunoglobulin concentrations are measured despite the fact that significant hypogammaglobulinemia could be a risk factor for increased mortality in critically ill patients (Shankar-Hari et al. 2015). Increased mortality was observed in immunoparalysis condition which can be recognized using relatively simple methods. It is reasonable to propose that septic patients are being monitored in terms of functioning of their immune system and potentially appropriate immunotherapy to be administered on the basis of specific results (Boomer et al. 2014; Leentjens et al. 2013; Nalos et al. 2012; Poujol et al. 2015).
Prerequisite for effective administration of immunotherapy is therefore the evaluation of immune system status and the degree of immunocompetence of individual patient. The example of such practice is French study on IFN-γ administration in sepsis preceded with biomarkers detection for identification of appropriate candidates for the treatment (Allantaz-Frager et al. 2013). The publication by Deutschman and Tracey suggest that severe sepsis and “persistent critical illness” are not immunopathologies “per se” but represent a failure of homeostasis caused by dysfunction of the neuroendocrine and immune system. Molecular and cellular mechanisms of this dysfunction are currently the focus of research and may open the door for new sepsis treatment modality (Deutschman and Tracey 2014).
Recently, newly updated definitions and clinical criteria for sepsis and septic shock, named ‘Sepsis-3’ have been published. One of the main objectives of ‘Sepsis-3’ is to simplify and clarify the terminology related to the syndrome, to improve consistency for epidemiology and research purposes, and to facilitate earlier recognition and more timely management of patients with, or at risk of developing sepsis (Shankar-Hari et al. 2016). A major change in new definitions is the elimination of SIRS and the term “severe sepsis”.
Due to the diversification and amplification of effector mechanisms of immunity, the blocking of proinflammatory cascades has little effect. The promising potential of genomics and proteomics has yet to be justified. Individualized sepsis therapy taking into account the state of the individual patient’s health seems to be a rational solution. However, the realistic expectations in mortality reduction are probably somewhere in the order of single-digit, rather than double-digit, percentages.
Molecular Biology in Sepsis Pathogenesis and Treatment Research
Another option in understanding sepsis pathogenesis, and thus in sepsis therapy is linked to the technologies enabling evaluation of gene expression and its regulatory mechanisms in addition to search for new proteins and metabolites produced in septic patients. Genomics, epigenetics, transcriptomics and metabolomics all represent such scientific quest (Fig. 2). In short summary, we are presenting basic information about these methods.
In 1988, Sorensen and colleagues demonstrated that the risk of dying from infectious disease was five times higher if an individual’s biological parent had also died of infectious disease (Sorensen et al. 1988). Since then, numerous studies have attempted to associate genetic markers of genomic variation (polymorphisms) with incidence or outcome of infectious disease and its sequelae in critically ill patients. Candidate genes for these association studies were chosen by utilizing knowledge of pathophysiologic pathways in infection and inflammation. Each step of such reaction may be affected by gene polymorphisms of many individual components of the immune system which will lead to susceptibility or resistance to infection. For instance, TNF gene polymorphisms showed association with an increased incidence as well as adverse outcomes in patients with severe sepsis and septic shock (Stuber et al. 1996). Specific allelic variants of the TNF locus are associated with increased susceptibility and adverse outcome of sepsis. Candidate gene studies have been extended towards pro- and anti-inflammatory cytokines like the IL-1 gene family, IL-6 and IL-10 (Fang et al. 1999). Genomic variants of candidate genes involved in pathogen recognition and signal transduction of inflammatory pathways like CD14 and TLRs may also contribute to incidence, severity and mortality of infectious complications in the critically ill (Hubacek et al. 2001; Mansur et al. 2015). In addition, it has been recognized that protein cascades involved in the pathophysiology of sepsis such as the coagulation cascade represent strong genomic candidate markers for association studies (Hermans et al. 1999). Latest findings from a European wide multicentre trial identified variants in the FER gene which are associated with a reduced risk of death from sepsis due to pneumonia (Rautanen et al. 2015). Genes activity varies due to different epigenetics mechanisms. The mechanisms involve DNA modification by methylation and histones modification. The main principle is gene expression variability without the change in DNA sequence (Phillips 2008). The example of this phenomenon is the bacteria-host interaction. Bacterial-induced epigenetic deregulations may affect host cell function either to promote host defense or to allow pathogen persistence. Thus, pathogenic bacteria can be considered as potential epimutagens able to reshape the epigenome (Bierne et al. 2012). Transcriptomics evaluates messenger RNA levels for genes in specific cells or tissue. Transcriptomics is used in diagnosis of sepsis for discrimination between infectious and noninfectious inflammation (Prucha et al. 2004; Tang et al. 2010). It can also identify biomarkers for the assessment of pathogenetic course and prognosis of the disease (Davenport et al. 2016). The main goal of proteomics is to identify proteins, biomarkers which are produced in septic patients, and thus can help in accurate diagnosis of sepsis, expression proteomics (Paugam-Burtz et al. 2010), or to detect proteins and to describe their function on the molecular level in sepsis—functional proteomics (Buhimschi et al. 2011). The term metabolome includes intra and extracellular low molecular substances which is the product of metabolic pathways needed for cell growth and its function. Metabolomics is complete analysis of metabolome in given physiological or pathological state of the cell, tissue and organism and it provides new insight on cellular fiction (Goodacre et al. 2004). It can be used in septic patients for diagnosis, disease prognosis and for the patient’s risk stratification (Ferrario et al. 2016; Kauppi et al. 2016).
Conclusion
Over the last two decades, experimental studies using cells and animals have greatly improved our knowledge of the pathophysiology of sepsis. The complexity of the host response and the multiple factors determining the outcome in patients with SIRS from infectious and noninfectious causes, as well as the failure of almost all adjunctive therapies aiming to interfere with individual components of this complex pathophysiology, have prompted to review the strengths and weaknesses of the current sepsis therapy. It was suggested that in the future, the management of patients with sepsis will necessitate more rigorous approaches to disease description and stratification (Abraham 2016; Bone 1996; Fink and Warren 2014).
Correct diagnosis, understanding of pathogenetic mechanisms and appropriate and timely therapy are needed for successful treatment of sepsis. For the immunotherapy to be rational and effective, the immunocompetence of a patient at a given stage of disease has to be evaluated. Using new technology in molecular biology—genomics, proteomics, transcriptomics and metabolomics is likely to achieve improvements in diagnostics and pathogenetic research of sepsis. This knowledge is very promising in terms of selection of appropriate immunotherapy for individual patient according to principles of personalized medicine. We believe that similar therapeutic approach as in hematooncology including targeted intervention on different levels of pathological process of patient’s immune system will be possible. For this reason, there is an urgent need to step up research and development activities in this area. Newly rediscovered PIRO (predisposition, infection, response, organ failure) classification system might be a better predictor of mortality in patients with sepsis (Granja et al. 2013; Macdonald et al. 2014).
Abbreviations
- G-CSF:
-
Granulocyte colony-stimulating factor
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- ICU:
-
Intensive care unit
- IL:
-
Interleukin
- IVIG:
-
Intravenous immunoglobulins
- LPS:
-
Lipopolysaccharide
- NF-κB:
-
Nuclear factor-κB
- PD-1:
-
Programmed death-1
- SIRS:
-
Systemic inflammatory response syndrome
- TLRs:
-
Toll-like receptors
- TNF-α:
-
Tumor necrosis factor α
References
Abraham E (2016) New definitions for sepsis and septic shock. Continuing evolution but with much still to be done. JAMA 315:757–759
Adhikari NK, Fowler RA, Bhagwanjee S et al (2010) Critical care and the global burden of critical illness in adults. Lancet 376:1339–1346
Adib-Conquy M, Monchi M, Goulenok C et al (2007) Increased plasma levels of soluble triggering receptor expressed on myeloid cells 1 and procalcitonin after cardiac surgery and cardiac arrest without infection. Shock 28:406–410
Alejandria MM, Lansang MA, Dans LF et al (2013) Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst Rev 9:CD001090
Allantaz-Frager F, Turrel-Davin F, Venet F et al (2013) Identification of biomarkers of response to IFNg during endotoxin tolerance: application to septic shock. PLoS One 8:e68218
Annane D, Seville V, Charpentier C et al (2002) Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288:862–871
Arndt P, Abraham E (2001) Immunological therapy of sepsis: experimental therapies. Intensive Care Med 27(Suppl 1):S104–S115
Berlot G, Vassallo MC, Busetto N et al (2012) Relationship between the timing of administration of IgM and IgA enriched immunoglobulins in patients with severe sepsis and septic shock and the outcome: a retrospective analysis. J Crit Care 27:167–171
Bhandari V, Wang C, Rinder C et al (2008) Hematologic profile of sepsis in neonates: neutrophil CD64 as a diagnostic marker. Pediatrics 121:129–134
Bierne H, Hamon M, Cossart P (2012) Epigenetics and bacterial infections. Cold Spring Harb Perspect Med 2:a010272
Bilgin K, Yaramiş A, Haspolat K et al (2001) A randomized trial of granulocyte- macrophage colony-stimulating factor in neonates with sepsis and neutropenia. Pediatrics 107:36–41
Bone RC (1996) Why sepsis trials fail. JAMA 276:565–566
Boomer JS, To K, Chang KC et al (2011) Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306:2594–2605
Boomer JS, Green JM, Hotchkiss RS (2014) The changing immune system in sepsis: is individualized immuno-modulatory therapy the answer? Virulence 5:45–56
Brahmamdam P, Inoue S, Unsinger J et al (2010) Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol 88:233–240
Buckley RH (2004) Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol 22:625–655
Buhimschi CS, Bhandari V, Dulay AT et al (2011) Proteomics mapping of cord blood identifies haptoglobin “switch-on” pattern as biomarker of early-onset neonatal sepsis in preterm newborns. PLoS One 6:e26111
Cao J, Xu F, Lin S et al (2014) IL-27 controls sepsis-induced impairment of lung antibacterial host defence. Thorax 69:926–937
Carpio R, Zapata J, Spanuth E et al (2015) Utility of presepsin (sCD14-ST) as a diagnostic and prognostic marker of sepsis in the emergency department. Clin Chim Acta 450:169–175
Carr R, Modi N, Doré CG (2003) CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst Rev 3:CD003066
Carr R, Brocklehurst P, Doré CJ et al (2009) Granulocyte-macrophage colony stimulating factor administered as prophylaxis for reduction of sepsis in extremely preterm, small for gestational age neonates (the PROGRAMS trial): a single-blind, multicentre, randomised controlled trial. Lancet 373:226–233
Cavaillon JM, Eisen D, Annane D (2014) Is boosting the immune system in sepsis appropriate? Crit Care 18:216
Corrêa TD, Jakob SM, Takala J (2015) Mitochondrial function in sepsis. Crit Care Horizons 1:31–41
Cronin L, Cook DJ, Carlet J et al (1995) Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med 23:1430–1439
Davenport EE, Burnham KL, Radhakrishnan J et al (2016) Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir Med 4:259–271
De Bosscher K, Van den Berghe W, Haegeman G (2003) The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev 24:488–522
Dejager L, Pinheiro I, Dejonckheere E et al (2011) Cecal ligation and puncture: the gold standard model for polymicrobial sepsis. Trends Microbiol 19:198–208
Dellinger RP, Levy MM, Rhodes A et al (2013) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637
Derkx B, Wittes J, McCloskey R (1999) Randomized, placebo-controlled trial of HA-1A, a human monoclonal antibody to endotoxin, in children with meningococcal septic shock. European Pediatric Meningococcal Septic Shock Trial Study Group. Clin Infect Dis 28:770–777
Deutschman CS, Tracey KJ (2014) Sepsis: current dogma and new perspective. Immunity 40:463–475
Döcke WD, Randow F, Syrbe U et al (1997) Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med 3:678–681
Echtenacher B, Urbaschek R, Weigl K et al (2003) Treatment of experimental sepsis induced immunoparalysis with TNF. Immunobiology 208:381–389
Elawady S, Botros SK, Sorour AE et al (2014) Neutrophil CD64 as a diagnostic marker of sepsis in neonates. J Investig Med 62:644–699
Esper A, Martin GS (2007) Is severe sepsis increasing in incidence and severity? Crit Care Med 35:1414–1415
Fang XM, Schroder S, Hoeft A et al (1999) Comparison of two polymorphisms of the interleukin-1 gene family: interleukin-1 receptor antagonist polymorphism contributes to susceptibility to severe sepsis. Crit Care Med 27:1330–1334
Ferrario M, Cambiaghi A, Brunelli L et al (2016) Mortality prediction in patients with severe septic shock: a pilot study using a target metabolomics approach. Sci Rep 6:20391
Fink MP, Warren HS (2014) Strategies to improve drug development for sepsis. Nat Rev Drug Discov 13:741–758
Fisher CJ Jr, Dhainaut JF, Opal SM et al (1994) Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 271:1836–1843
Goodacre R, Vaidyanathan S, Dunn WB et al (2004) Metabolomics by numbers: acquiring and understanding global metabolite data. Trends Biotechnol 22:245–252
Granja C, Povoa P, Lobo C et al (2013) The predisposition, infection, response and organ failure (piro) sepsis classification system: results of hospital mortality using a novel concept and methodological approach. PLoS One 8:e53885
Hafezi-Moghadam A, Simonini T, Yang Z et al (2002) Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med 8:473–479
Hebbar PB, Archer TK (2003) Chromatin remodeling by nuclear receptors. Chromosoma 111:495–504
Hentrich M, Fehnle K, Ostermann H et al (2006) IgMA-enriched immunoglobulin in neutropenic patients with sepsis syndrome and septic shock: a randomized, controlled, multiple-center trial. Crit Care Med 34:1319–1325
Hermans PW, Hibberd ML, Booy R et al (1999) 4G/5G promoter polymorphism in the plasminogen-activator-inhibitor-1 gene and outcome of meningococcal disease. Meningococcal Research Group. Lancet 354:556–560
Herzig DS, Guo Y, Fang G et al (2012) Therapeutic efficacy of CXCR3 blockade in experimental model of severe sepsis. Crit Care 16:R168
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150
Hotchkiss RS, Monneret G, Payen D (2013) Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13:260–268
Huang W, Tang Y, Li L et al (2010) HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine 51:119–126
Hubacek JA, Stuber F, Frohlich D et al (2001) Gene variants of the bactericidal/permeability increasing protein and lipopolysaccharide binding protein in sepsis patients: gender-specific genetic predisposition to sepsis. Crit Care Med 29:557–561
Icardi M, Erickson Y, Kilborn S et al (2009) CD64 index provides simple and predictive testing for detection and monitoring of sepsis and bacterial infection in hospital patients. J Clin Microbiol 47:3914–3919
INIS Collaborative Group, Brocklehurst P, Farrell B, King A et al (2011) Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med 365:1201–1211
Inoue S, Unsinger J, Davis CG et al (2010) IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improve survival in sepsis. J Immunol 184:1401–1409
Ito R, Takahashi T, Katano I et al (2012) Current advances in humanized mouse models. Cell Mol Immunol 9:208–214
Jawad I, Luksic I, Rafnsson SB (2012) Assesing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health 2:010404
Kaneider NC, Agarwal A, Leger AJ et al (2005) Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat Med 11:661–665
Karzai W, von Specht BU, Parent C et al (1999) G-CSF during Escherichia coli versus Staphylococcus aureus pneumonia in rats has fundamentally different and opposite effects. Am J Respir Crit Care Med 159(5 Pt 1):1377–1382
Kaukonen KM, Bailey M, Suzuki S et al (2014) Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 311:1308–1311
Kauppi AM, Edin A, Ziegler I et al (2016) Metabolites in blood for prediction of bacteremic sepsis in the emergency room. PLoS ONE 11:e0147670
Koh GC, Weehuizen TA, Breitbach K et al (2013) Glyburide reduces bacterial dissemination in a mouse model of melioidosis. PLoS Negl Trop Dis 7:e2500
Kreymann KG, de Heer G, Nierhaus A et al (2007) Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit Care Med 35:2677–2685
Lederer JA, Rodrick ML, Mannick JA (1999) The effects of injury on the adaptive immune response. Shock 11:153–159
Leentjens J, Kox M, Koch RM et al (2012) Reversal of immunoparalysis in humans in vivo: a double-blind, placebo-controlled, randomized pilot study. Am J Respir Crit Care Med 186:838–845
Leentjens J, Kox M, van der Hoeven JG et al (2013) Immunotherapy for the adjunctive treatment of sepsis: from immunosuppression to immunostimulation. Time for a paradigm change? Am J Respir Crit Care Med 187:1287–1293
Lin CW, Lo S, Perng DS et al (2014) Complete activation of autophagic process attenuates liver injury and improves survival in septic mice. Shock 41:241–249
Liotta LA, Kohn EC, Petricoin EF (2001) Clinical proteomics: personalized molecular medicine. JAMA 286:2211–2214
Liotta LA, Ferrari M, Petricoin EF (2003) Clinical proteomics: written in blood. Nature 425:905
Lorente JA, Marshall JC (2005) Neutralization of tumor necrosis factor in preclinical models of sepsis. Shock 24(Suppl 1):107–119
Macdonald SP, Arendts G, Fatovich DM et al (2014) Comparison of PIRO, SOFA, and MEDS scores for predicting mortality in emergency department patients with severe sepsis and septic shock. Acad Emerg Med 21:1257–1263
Mansur A, Liese B, Steinau M et al (2015) The CD14 rs2569190 TT genotype is associated with an improved 30-days survival in patients with sepsis: a prospective observational study. PLoS One 10:e0127761
Marik PE, Pastores SM, Annane D, American College of Critical Care Medicine et al (2008) Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med 36:1937–1949
Marioli A, Koupetori M, Raftogianis M et al (2014) Early ganges of the kinetics of monocyte trem-1 reflect final outcome in human sepsis. BMC Immunol 15:585–593
Martin GS (2012) Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther 10:701–706
Martin G, Brunkhorst FM, Janes JM et al (2009) The international PROGRESS registry of patients with severe sepsis: drotrecogin alfa (activated) use and patient outcomes. Crit Care 13:R103
Matsumura H, Onozuka K, Terada Y et al (1990) Effect of murine recombinant interferon-gamma in the protection of mice against Salmonella. Int J Immunopharmacol 12:49–56
McCloskey RV, Straube RC, Sanders C et al (1994) Treatment of septic shock with human monoclonal antibody HA-1A. A randomized, double-blind, placebo-controlled trial. CHESS Trial Study Group. Ann Intern Med 121:1–5
Medzhitov R, Schneider DS, Soares MP (2012) Disease tolerance as a defense strategy. Science 335:936–941
Melican K, Dumeni G (2013) Humanized model of microvascular infection. Future Microbiol 8:567–569
Morel J, Singer M (2014) Statins, fibrates, thiazolidinediones and resveratrol as adjunctive therapies in sepsis: could mitochondria be a common target? Intensive Care Med Exp 2:9
Nakos G, Malamou-Mitsi VD, Lachtana A et al (2002) Immunoparalysis in patients with severe trauma and the effect of inhaled interferon-gamma. Crit Care Med 30:1488–1494
Nalos M, Santner-Nanan B, Parnell G et al (2012) Immune effects of interferon gamma in persistent staphylococcal sepsis. Am J Respir Crit Care Med 185:110–112
Nelson S, Belknap SM, Carlson RW et al (1998) A randomized controlled trial of filgrastim as an adjunct to antibiotics for treatment of hospitalized patients with community-acquired pneumonia. CAP Study Group. J Infect Dis 178:1075–1080
Nuutila J (2010) The novel applications of the quantitative analysis of neutrophil cell surface FcgammaRI (CD64) to the diagnosis of infectious and inflammatory diseases. Curr Opin Infect Dis 23:268–274
Ohlsson A, Lacy JB (2004) Intravenous immunoglobulin for suspected or subsequently proven infection in neonates. Cochrane Database Syst Rev 1:001239
Opal SM, Fisher CJ Jr, Dhainaut JF et al (1997) Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med 25:1115–1124
Opal SM, Laterre PF, Francois B et al (2013) Effect of Eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis. The ACCESS randomized trial. JAMA 309:1154–1162
Orozco H, Arch J, Medina-Franco H et al (2006) Molgramostim (GM-CSF) associated with antibiotic treatment in nontraumatic abdominal sepsis: a randomized, double-blind, placebo-controlled clinical trial. Arch Surg 141:150–153
Paugam-Burtz C, Albuquerque M, Baron G et al (2010) Plasma proteome to look for diagnostic biomarkers of early bacterial sepsis after liver transplantation: a preliminary study. Anesthesiology 112:926–935
Pena OM, Hancock DG, Lyle NH et al (2014) An endotoxin tolerance signature predicts sepsis and organ dysfunction at initial clinical presentation. EBioMedicine 1:64–71
Penack O, Becker C, Buchheidt D et al (2014) Management of sepsis in neutropenic patients: 2014 updated guidelines from the Infectious Diseases Working Party of the German Society of Hematology and Medical Oncology (AGIHO). Ann Hematol 93:1083–1095
Phillips T (2008) The role of methylation in gene expression. Nat Educ 1:1–6
Poujol F, Monneret G, Pachot A et al (2015) Altered T lymphocyte proliferation upon lipopolysaccharide challenge ex vivo. PLoS One 10:e0144375
Presneill JJ, Hartus T, Stewart AG et al (2002) A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am J Respir Crit Care Med 166:138–143
Prucha M, Ruryk A, Boriss H et al (2004) Expression profiling: toward an application in sepsis diagnostics. Shock 22:29–33
Qiang X, Yang WL, Wu R et al (2013) Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med 19:1489–1495
Raithatha AH, Bryden DC (2012) Use of intravenous immunoglobulin therapy in the treatment of septic shock, in particular severe invasive group A streptococcal disease. Indian J Crit Care Med 16:37–40
Rankin JS, Oguntolu O, Binford RS et al (2011) Management of immune dysfunction after adult cardiac surgery. J Thorac Cardiovasc Surg 142:575–580
Rautanen A, Mills TC, Gordon AC et al (2015) Genome-wide association study of survival from sepsis due to pneumonia: an observational cohort study. Lancet Respir Med 3:53–60
Reutershan J, Stockton R, Zarbock A et al (2007) Blocking p21-activated kinase reduces lipopolysaccharide-induced acute lung injury by preventing polymorphonuclear leukocyte infiltration. Am J Respir Crit Care Med 175:1027–1035
Rittirsch D, Hoesel LM, Ward PA (2007) The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol 81:137–143
Root RK, Lopato RF, Patrick W, Pneumonia Sepsis Study Group et al (2003) Multicenter, double-blind, placebo-controlled study of the use of filgrastim in patients hospitalized with pneumonia and severe sepsis. Crit Care Med 31:367–373
Rosenbloom AJ, Linden PK, Dorrance A et al (2005) Effect of granulocyte-monocyte colony-stimulating factor therapy on leukocyte function and clearance of serious infection in nonneutropenic patients. Chest 127:2139–2150
Russwurm S, Reinhart K (2004) Procalcitonin mode of action: new pieces in a complex puzzle. Crit Care Med 32:1801–1802
Schefold JC (2011) Immunostimulation using granulocyte- and granulocyte macrophage colony stimulating factor in patients with severe sepsis and septic shock. Crit Care 15:136
Seok J, Warren HS, Cuenca AG et al (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110:3507–3512
Sevransky JE, Parent C, Cui X et al (2004) Granulocyte colony-stimulating factor has differing effects comparing intravascular versus extravascular models of sepsis. J Trauma 57:618–625
Sewnath ME, Olszyna DP, Birjmohun R et al (2001) IL-10-deficient mice demonstrate multiple organ failure and increased mortality during Escherichia coli peritonitis despite an accelerated bacterial clearance. J Immunol 166:6323–6331
Shankar-Hari M, Culshaw N, Post B et al (2015) Endogenous IgG hypogammaglobulinemia in critically ill adults with sepsis: review and meta-analysis. Intensive Care Med 41:1393–1401
Shankar-Hari M, Phillips GS, Levy ML et al (2016) Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:775–787
Shay T, Jojic V, Zuk O et al (2013) Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc Natl Acad Sci USA 110:2946–2951
Shindo Y, Unsinger J, Burnham CA et al (2015) Interleukin-7 and anti-programmed cell death 1 antibody have differing effects to reverse sepsis-induced immunosuppression. Shock 43:334–343
Simon L, Gauvin F, Amre DK et al (2004) Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analyses. Clin Infect Dis 39:206–217
Singer M (2014) The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 5:66–72
Sorensen TIA, Nielsen GG, Anderson PK et al (1988) Genetic and environmental influences on premature death in adult adoptees. N Engl J Med 318:727–732
Sprung CL, Annane D, Keh D et al (2008) Hydrocortisone therapy for patients with septic shock. N Engl J Med 358:111–124
Stuber F, Petersen M, Bokelmann F et al (1996) A genomic polymorphism with in the tumor necrosis factor locus influences plasma tumor necrosis factor concentrations and outcome of patients with severe sepsis. Crit Care Med 24:381–384
Tang BM, Eslick GD, Craig JC et al (2007) Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis 7:210–217
Tang BM, Huang SJ, McLean AS (2010) Genome-wide transcription profiling of human sepsis: a systematic review. Crit Care 14:R237
Tracey KJ, Fong Y, Hesse DG et al (1987) Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330:662–664
Turgeon AF, Hutton B, Fergusson DA et al (2007) Meta-analysis: intravenous immunoglobulin in critically ill adult patients with sepsis. Ann Intern Med 146:193–203
Ulloa L, Ochani M, Yang H et al (2002) Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci USA 99:12351–12356
Unsinger J, McGlynn M, Kasten KR et al (2010) IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol 184:3768–3779
Vincent JL, Sakr Y, Sprung CL et al (2006) Sepsis occurrence in acutely ill patients. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34:344–353
Walker MA, Volpi S, Sims KB et al (2014) Powering the immune system: mitochondria in immune function and deficiency. J Immunol Res 2014:164309
Welte T, Dellinger RP, Ebelt H et al (2015) Concept for a study design in patients with severe community-acquired pneumonia: a randomised controlled trial with a novel IGM-enriched immunoglobulin preparation. The CIGMA study. Respir Med 109:758–767
Werdan K, Pilz G, Bujdoso O et al (2007) Score-Based Immunoglobulin Therapy of Sepsis (SBITS) Study Group: score-based immunoglobulin G therapy of patients with sepsis: the SBITS study. Crit Care Med 35:2693–2701
Wesche-Soldato DE, Chung CS, Lomas-Neira J et al (2005) In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood 106:2295–2301
Xing Z, Gauldie J, Cox G et al (1998) IL-6 is an anti-inflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest 101:311–320
Yaegashi Y, Shirakawa K, Sato N et al (2005) Evaluation of a newly identified soluble CD14 subtype as a marker of sepsis. J Infect Chemother 11:234–238
Yang H, Ochani M, Li J et al (2004) Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA 101:296–301
Zhang X, Liu D, Liu YN et al (2015) The accuracy of presepsin (sCD14-ST) for the diagnosis of sepsis in adults: a meta-analysis. Crit Care 19:323–334
Zheng G, Lyu J, Huang J et al (2015) Experimental treatment for mitochondrial dysfunction in sepsis: a narrative review. J Res Med Sci 20:185–195
Ziegler EJ, Fisher CJ Jr, Sprung CL et al (1991) Treatment of Gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med 324:429–436
Zou L, Feng Y, Zhang M et al (2013) Complement factor B is the downstream effector of TLRs and plays an important role in a mouse model of severe sepsis. J Immunol 191:5625–5635
Acknowledgments
This work was supported by the Czech Republic Ministry of Health's conceptual development of research organisation (Hospital Na Homolce—NNH, 00023884, IG144101 and Thomayer Hospital—TN, 00064190).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose no conflicts of interest.
About this article
Cite this article
Prucha, M., Zazula, R. & Russwurm, S. Immunotherapy of Sepsis: Blind Alley or Call for Personalized Assessment?. Arch. Immunol. Ther. Exp. 65, 37–49 (2017). https://doi.org/10.1007/s00005-016-0415-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-016-0415-9