Abstract
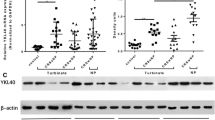
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a common disease that has a considerable impact on the quality of life. Alterations in signalling pathways may contribute to the ongoing inflammation and proliferation in CRSwNP. The MEK1/2–ERK1/2 pathway transmits signals from many extracellular molecules to regulate cellular processes. We examined tissue samples from nasal polyps and the inferior turbinate of patients with CRSwNP and the inferior turbinate from subjects with healthy mucosa. The expressions of MEK1/2, ERK1/2, and their active phosphorylated forms pMEK1/2 and pERK1/2 were analysed using DNA microarray, quantitative real-time PCR, protein array, Western hybridisation, and immunohistochemistry. We detected increased MEK1/2 protein expression in nasal polyps compared to the inferior turbinates of patients with CRSwNP or healthy mucosa. We also found a higher amount of MEK1/2 in the inferior turbinates of patients with CRSwNP compared to those with healthy mucosa. Most importantly, we observed a significant increase in the phosphorylation of MEK1/2 and ERK1/2 in nasal polyps compared to both types of controls. We observed activation of the MEK1/2–ERK1/2 pathway in nasal polyps. Interestingly, we did not see the same activation pattern in different tiers of the MEK1/2–ERK1/2 signalling cascade. One explanation for this result is that the components enhance the complex MEK–ERK cascade in a distinct manner, enabling a wide variety of functions. The MEK1/2–ERK1/2 pathway appears to play a pivotal role in the pathogenesis of CRSwNP.






Similar content being viewed by others
References
Anderson CN, Tolkovsky AM (1999) A role for MAPK/ERK in sympathetic neuron survival: protection against a p53-dependent, JNK-independent induction of apoptosis by cytosine arabinoside. J Neurosci 19:664–673
Bachert C, Zhang N (2012) Chronic rhinosinusitis and asthma: novel understanding of the role of IgE “above atopy”. J Intern Med 272:133–143
Baumann I, Plinkert PK, De Maddalena H (2008) Development of a grading scale for the Sino-Nasal Outcome Test-20 German Adapted Version (SNOT-20 GAV). HNO 56:784–788
Boulton TG, Gregory JS, Cobb MH (1991a) Purification and properties of extracellular signal-regulated kinase 1, an insulin-stimulated microtubule-associated protein 2 kinase. Biochemistry 30:278–286
Boulton TG, Nye SH, Robbins DJ et al (1991b) ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 65:663–675
Buckley S, Driscoll B, Barsky L et al (1999) ERK activation protects against DNA damage and apoptosis in hyperoxic rat AEC2. Am J Physiol 277(1 Pt 1):L159–L166
Cargnello M, Roux PP (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 75:50–83
Chang WC, Lee YC, Liu CL et al (2001) Increased expression of iNOS and c-fos via regulation of protein tyrosine phosphorylation and MEK1/ERK2 proteins in terminal bronchiole lesions in the lungs of rats exposed to cigarette smoke. Arch Toxicol 75:28–35
Cowley S, Paterson H, Kemp P et al (1994) Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 77:841–852
Crews CM, Alessandrini A, Erikson RL (1992) The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science 258:478–480
Dhillon AS, Hagan S, Rath O et al (2007) MAP kinase signalling pathways in cancer. Oncogene 26:3279–3290
Edelman AM, Blumenthal DK, Krebs EG (1987) Protein serine/threonine kinases. Annu Rev Biochem 56:567–613
Erhardt P, Schremser EJ, Cooper GM (1999) B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol Cell Biol 19:5308–5315
Fokkens WJ, Lund VJ, Mullol J et al (2012) European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl 23:1–298
Foncea R, Gálvez A, Pérez V et al (2000) Extracellular regulated kinase, but not protein kinase C, is an antiapoptotic signal of insulin-like growth factor-1 on cultured cardiac myocytes. Biochem Biophys Res Commun 273:736–744
Fukuda M, Gotoh I, Gotoh Y et al (1996) Cytoplasmic localization of mitogen-activated protein kinase directed by its NH2-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. J Biol Chem 271:20024–20028
Furuya A, Asano K, Shoji N et al (2010) Suppression of nitric oxide production from nasal fibroblasts by metabolized clarithromycin in vitro. J Inflamm 7:56
Garavello W, Viganò P, Romagnoli M et al (2005) Expression of cell cycle regulatory proteins and analysis of apoptosis in normal nasal mucosa and in nasal polyps. Am J Rhinol 19:549–553
Hirotsu M, Kikuchi K, Kusunoki T et al (2011) Comparison of bacterial examinations between eosinophilic and neutrophilic chronic rhinosinusitis with nasal polyps. Acta Otolaryngol 131:997–1001
Jaaro H, Rubinfeld H, Hanoch T et al (1997) Nuclear translocation of mitogen-activated protein kinase kinase (MEK1) in response to mitogenic stimulation. Proc Natl Acad Sci USA 94:3742–3747
Kim SJ, Ju JW, Oh CD et al (2002) ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J Biol Chem 277:1332–1339
Kitagawa D, Tanemura S, Ohata S et al (2002) Activation of extracellular signal-regulated kinase by ultraviolet is mediated through Src-dependent epidermal growth factor receptor phosphorylation. Its implication in an anti-apoptotic function. J Biol Chem 277:366–371
Le Gall M, Chambard JC, Breittmayer JP et al (2000) The p42/p44 MAP kinase pathway prevents apoptosis induced by anchorage and serum removal. Mol Biol Cell 11:1103–1112
Lin SK, Kok SH, Shun CT et al (2007) Tumor necrosis factor-alpha stimulates the expression of C–C chemokine ligand 2 gene in fibroblasts from the human nasal polyp through the pathways of mitogen-activated protein kinase. Am J Rhinol 21:251–255
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 25:402–408
Lloyd AC (2006) Distinct functions for ERKs? J Biol 5:13
Lu Z, Xu S (2006) ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 58:621–631
Lu Z, Xu S, Joazeiro C et al (2002) The PHD domain of MEKK1 acts as an E3 ubiquitin ligase and mediates ubiquitination and degradation of ERK1/2. Mol Cell 9:945–956
Lund VJ, Mackay IS (1993) Staging in rhinosinusitus. Rhinology 31:183–184
Mercer BA, D’Armiento JM (2006) Emerging role of MAP kinase pathways as therapeutic targets in COPD. Int J Chron Obstruct Pulmon Dis 1:137–150
Mercer BA, Kolesnikova N, Sonett J et al (2004) Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase-1 induction by cigarette smoke. J Biol Chem 279:17690–17696
Nagata Y, Todokoro K (1999) Requirement of activation of JNK and p38 for environmental stress-induced erythroid differentiation and apoptosis and of inhibition of ERK for apoptosis. Blood 94:853–863
Pace E, Scafidi V, Di Bona D et al (2012) Increased expression of IL-19 in the epithelium of patients with chronic rhinosinusitis and nasal polyps. Allergy 67:878–886
Pacova H, Astl J, Martinek J (2009) The pathogenesis of chronic inflammation and malignant transformation in the human upper airways: the role of beta-defensins, eNOS, cell proliferation and apoptosis. Histol Histopathol 24:815–820
Piccirillo JF, Merritt MG Jr, Richards ML (2002) Psychometric and clinimetric validity of the 20-Item Sino-Nasal Outcome Test (SNOT-20). Otolaryngol Head Neck Surg 126:41–47
Pulido R, Zúñiga A, Ullrich A (1998) PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. EMBO J 17:7337–7350
Ratjen F, Döring G (2003) Cystic fibrosis. Lancet 361:681–689
Riechelmann H, Deutschle T, Rozsasi A et al (2005) Nasal biomarker profiles in acute and chronic rhinosinusitis. Clin Exp Allergy 35:1186–1191
Rosen OM (1987) After insulin binds. Science 237:1452–1458
Roskoski R Jr (2012) MEK1/2 dual-specificity protein kinases: structure and regulation. Biochem Biophys Res Commun 417:5–10
Rossomando AJ, Payne DM, Weber MJ et al (1989) Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci USA 86:6940–6943
Rubinfeld H, Seger R (2005) The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol 31:151–174
Sachse F, Becker K, von Eiff C et al (2010) Staphylococcus aureus invades the epithelium in nasal polyposis and induces IL-6 in nasal epithelial cells in vitro. Allergy 65:1430–1437
Schubert MS (2004) Allergic fungal sinusitis. Otolaryngol Clin North Am 37:301–326
Seger R, Krebs EG (1995) The MAPK signaling cascade. FASEB J 9:726–735
Seger R, Seger D, Lozeman FJ et al (1992) Human T-cell mitogen-activated protein kinase kinases are related to yeast signal transduction kinases. J Biol Chem 267:25628–25631
Seger R, Seger D, Reszka AA et al (1994) Overexpression of mitogen-activated protein kinase kinase (MAPKK) and its mutants in NIH 3T3 cells. Evidence that MAPKK involvement in cellular proliferation is regulated by phosphorylation of serine residues in its kinase subdomains VII and VIII. J Biol Chem 269:25699–25709
Sejima T, Holtappels G, Kikuchi H et al (2012) Cytokine profiles in Japanese patients with chronic rhinosinusitis. Allergol Int 61:115–122
Sun Y, Zhou B, Wang CS et al (2012) Clinical and histopathologic features of biofilm-associated chronic rhinosinusitis with nasal polyps in Chinese patients. Chin Med J 125:1104–1109
Szelenyi ER, Urso ML (2012) Time-course analysis of injured skeletal muscle suggests a critical involvement of ERK1/2 signaling in the acute inflammatory response. Muscle Nerve 45:552–561
Tanoue T, Adachi M, Moriguchi T et al (2000) A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol 2:110–116
Tóth L, Csomor P, Sziklai I et al (2011) Biofilm detection in chronic rhinosinusitis by combined application of hematoxylin–eosin and gram staining. Eur Arch Otorhinolaryngol 268:1455–1462
Tran SE, Holmstrom TH, Ahonen M et al (2001) MAPK/ERK overrides the apoptotic signaling from Fas, TNF, and TRAIL receptors. J Biol Chem 276:16484–16490
Van Zele T, Claeys S, Gevaert P et al (2006) Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 61:1280–1289
Vantaggiato C, Formentini I, Bondanza A et al (2006) ERK1 and ERK2 mitogen-activated protein kinases affect Ras-dependent cell signaling differentially. J Biol 5:14
Wang X, Martindale JL, Liu Y et al (1998) The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J 333(Pt 2):291–300
Weng L, Dai H, Zhan Y et al (2006) Rosetta error model for gene expression analysis. Bioinformatics 22:1111–1121
Widmann C, Gibson S, Jarpe MB et al (1999) Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev 79:143–180
Yoon S, Seger R (2006) The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 24:21–44
Zheng CF, Guan KL (1994) Activation of MEK family kinases requires phosphorylation of two conserved Ser/Thr residues. EMBO J 13:1123–1131
Acknowledgments
We are grateful to Brigitte Wollmann and Birgit Hüsing for their skilful support in several parts of this work and to all of the members of the Department of Otorhinolaryngology for their helpful discussions and a collegial atmosphere. There are no conflicts of interest. This work was supported by the Klosterfrau GmbH, Köln, Germany.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Linke, R., Pries, R., Könnecke, M. et al. The MEK1/2–ERK1/2 Pathway is Activated in Chronic Rhinosinusitis with Nasal Polyps. Arch. Immunol. Ther. Exp. 62, 217–229 (2014). https://doi.org/10.1007/s00005-014-0281-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-014-0281-2