Abstract
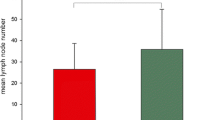
PURPOSE: Lymph-node involvement is the most important prognostic factor in colorectal cancers. Many staging systems adopted node status as a parameter of tumor classification. However, the number of identified and positive glands varies across articles, depending on specimen examination. There is a consistent risk of substaging tumors and undertreating patients. Aim of this study was to investigate the prognostic significance of different pathologic methods. METHODS: Eight hundred one patients who underwent curative resection of colorectal cancer entered the study and were divided into two groups. In Group 1 the specimen was “en bloc” fixed, and nodes were identified by sight and palpation. In Group 2 the mesentery of the excised specimen was dissected away from the bowel, stretched, and pinned to cork board. The mesenteric segment surrounding the origin of principal vessels was divided from the segment surrounding the colic vessels. All specimen segments were fixed, node identification being performed by sight and palpation. Examined and positive nodes were recorded, and metastatic rate and incidence was calculated in the two groups. Patients were classified with used of different staging systems. Survival rates were calculated, related to tumor stage, and compared statistically. Pathologic procedures were included in a multivariate analysis. RESULTS: A significantly higher number of detected and positive nodes and metastatic rate (37.5vs. 30.2 percent;P<0.05) were observed in Group 2; 45.2 percent of Group 2 and 25.3 percent of Group 1 cases had more than three positive nodes (P<0.05). In Group 2 several patients shifted from earlier to more advanced stages compared with Group 1 cases. Five-year and ten-year survival rates were significantly higher (P=p.pr) in Group 2 (81.5 and 77.2 percent) than in Group 1 (76.7 and 61.5 percent), mostly in patients with TNM Stage N0. Survival analysis related to Astler and Coller's and Tang's classifications confinrmed such features. Higher rates of local recurrences and distant metastases were found in Group 1, particularly if related to node status (P<0.05). Multivariate analysis demonstrated the pathologic method is an independent prognostic factor. CONCLUSIONS: This study demonstrates the prognostic impact of specimen examination. Inaccurate methods could downstage the tumor and exclude the patient from adjuvant therapies, with detrimental effects on the outcome of the case.
Similar content being viewed by others
References
Carlon CA, Fabris G, Arslan-Pagnini C, Pluchinotta AM, Chinelli E, Carniato S. Prognostic correlation of operable carcinoma of the rectum. Dis Colon Rectum 1985;28:47–50.
Beahrs O. Colorectal cancer staging as prognostic feature. Cancer 1982;50:2615–7.
Hermanek P, Altendorf A, Classification of colorectal carcinoma with regional lymphatic metastasis. Pathol Res Pract 1981;173:1–11.
Brandao O, Sabrinho-Simoe S, Serrao D. Prognosis in colorectal carcinoma. Pathol Res Pract 1985;180:506–10.
Third Report of the M. R. C. Trial on behalf of the working Party: Clinico pathological features of prognostic significance in operable rectal cancer in 17 centres in the United Kingdom. Br J Cancer 1984;50:435-42.
Steinberg SM, Barwich KW, Stablein DM. Importance of tumor pathology and morphology in patients with surgically resected colon cancer. Findings from the Gastrointestinal Tumor Study Group. Cancer 1986;58:1340–5.
Copeland M, Milleri D, Jones RS. Prognostic factors in carcinoma of the colon and rectum. Am J Surg 1968;116:875–81.
Grinnel RS. Lymphatic block with atypical and retrograde lymphatic metastasis and spread on carcinoma of the colon and rectum. Ann Surg 1966;163:272–80.
Hojo K, Koyama Y. The effectiveness of wide anatomical resection and radical lymphadenectomy for patients with rectal cancer. Jpn J Surg 1982;12:111–6.
Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg 1954;139:846–52.
Wolmark N, Fisher B, Wieand HS. The prognostic value of the modification of the Dukes C class of colorectal cancer. An analysis of the NSABP TRIAL. Ann Surg 1986;139:846–52.
Shepherd JM, Johnes FS. Adenocarcinoma of the large bowel. Br J Cancer 1971;25:680–4.
Faltermann KW, Hill CB, Markey JC, Fox JW, Cohn I Jr. Cancer of the colon-rectum and anus. Cancer 1974;34:951–9.
Rubin P. Current concepts in cancer. JAMA 1975;231:513–8.
Hojo K, Koyama Y, Moriya Y. Lymphatic spread and its prognostic value in patients with rectal cancer. Am J Surg 1982;144:350–4.
Schmitz-Moormann P, Himmelmann GW, Baum U, Nilles M. Morphological predictors of survival in colorectal carcinoma: univariate and multivariate analysis. J Cancer Res Clin Oncol 1987;113:586–92.
Gabriel WB, Dukes CE, Bussey HJ. Lymphatic spread in carcinoma of the rectum. Surg Gynecol Obstet 1967;143:793–4.
Dukes CE. The classification of cancer of the colon. J Pathol 1982;35:323–32.
Sugarbaker PH. Carcinoma of the colon: prognosis and operative procedure. Curr Probl Surg 1981;18:791–4.
First Report of a Working Party. A trial of pre-operative radiotherapy in the management of operable rectal cancer. Br J Surg 1982;69:513-9.
Gastro Intestinal Tumour Study Group. Adjuvant therapy of colon cancer: results of a prospective randomized trial. N Engl J Med 1984;310:737–43.
Chapuis PH, Dent OF, Fisher R,et al. A multivariate analysis of clinical and pathological variables in prognosis of resection of large bowel cancer. Br J Surg 1985;72:698–702.
Tang R, Wang JY, Chen JS,et al. Survival impact of lymph node metastasis in TNM stage III carcinoma of the colon and rectum. J Am Coll Surg 1995;180:705–12.
Gunderson LL, and Sosin H. Areas of failure found at reoperation (second or symptomatic look) following “curative resection” for adenocarcinoma of the rectum. Cancer 1974;34:1278–92.
Hermanek P, Sobin LH (UICC), eds. TNM Classification of Malignant Tumors. 4th ed. Berlin: Springer-Verlag, 1987:47–49.
Crucitti F, Doglietto GB, Bellantone R,et al. Accurate specimen preparation and examination is mandatory to detect lymph nodes and avoid understaging in colorectal cancer. J Surg Oncol 1992;51:153–7.
Wand W, Jones MC. Kernel smoothing. London: Chapman and Hall, 1995.
Sofo L, Ratto C, Doglietto GB,et al. Intraoperative radiation therapy in integrated treatment of rectal cancers: results of Phase II study. Dis Colon Rectum 1996;39:1396–403.
Cawthorn SJ, Gibbs NM, Marks CG. Clearance technique for the detection of lymph nodes in colorectal cancer. Br J Surg 1986;73:58–60.
Herrera-Ornelas L, Justiniano J, Castillo N, Petrelli NJ, Stulc JP, Mittelman A. Metastases in small lymph nodes from colon cancer. Arch Surg 1987;122:1253–6.
Herrera L, Villareal JR. Incidence of metastases from rectal adenocarcinoma in small lymph nodes detected by a clearing technique. Dis Colon Rectum 1992;35:783–8.
Hida J, Mori N, Kubo R,et al. Metastases from carcinoma of the colon and rectum detected in small lymph nodes by the clearing method. J Am Coll Surg 1994;178:223–8.
Scott KW, Grace RH. Detection of lymph node metastases in colorectal carcinoma before and after fat clearance. Br J Surg 1989;76:1165–7.
Coller FA, Kay EB, MacIntire RS. Regional lymphatic metastases of carcinoma of the colon [letter]. Ann Surg 1941;114:56.
Gibbs NM. Large paraffin sections and chemical clearance of axillary tissues as a routine procedure in the pathological examination of the breast. Histopathology 1982;6:647–60.
Dukin K, Haagensen CD. An improved technique for the study of lymph nodes in surgical specimens. Ann Surg 1980;191:419–29.
Pickren JW. Nodal clearance and detection. JAMA 1975;231:969–71.
Gilchrist RK, David VC. Lymphatic spread of carcinoma of the rectum. Ann Surg 1938;10:621–42.
Schmita-Moorman P, Thomas C, Pohl C, Sohl R. Pathoanatomical demonstration of lymph node metastases in a surgical specimen. Path Res Pract 1982;174:403–11.
Gilchrist RK, David VC. A consideration of pathological factors influencing five year survival in radical resection of the large bowel and rectum for carcinoma. Ann Surg 1947;126:421–38.
Grinnell RS. Lymphatic metastases of carcinoma of the colon and rectum. Ann Surg 1950;131:494–506.
Hyder JW, Talbott TM, Maycroft TC. A critical review of chemical lymph node clearance and staging of colon and rectal cancer at Ferguson Hospital, 1977 to 1982. Dis Colon Rectum 1990;33:923–5.
Haboubi NY, Clark P, Kaftan SM, Schofield PF. The importance of combining xylene clearance and immunohistochemistry in the accurate staging of colorectal carcinoma. J R Soc Med 1992;85:386–8.
Greeson JK, Isenhart CE, Rice R, Mojzisik C, Houchens D, Martin EW. Identification of occult micrometastases in pericolic lymph nodes of Dukes B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Cancer 1994;73:563–9.
Franke WW, Moll R. Cytoskeletal components of lymphoid organs. Differentiation 1987;36:145–63.
Haboubi NY, Clark P. Cytokeratin in plasmacytomas [letter]. Histopathology 1990;17:482.
Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med 1985;312:1604–8.
Author information
Authors and Affiliations
Additional information
Supported by the Italian National Research Council (C.N.R.) with Grant #97.04080.CT04.
About this article
Cite this article
Ratto, C., Sofo, L., Ippoliti, M. et al. Accurate lymph-node detection in colorectal specimens resected for cancer is of prognostic significance. Dis Colon Rectum 42, 143–154 (1999). https://doi.org/10.1007/BF02237119
Issue Date:
DOI: https://doi.org/10.1007/BF02237119