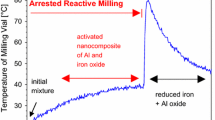
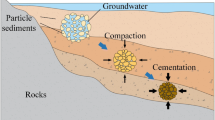
Abstract
Reviews of aqueous processing in JOM have traditionally focused on hydrometallurgical process routes. This article, however, addresses the application of aqueous processing in materials engineering and presents some promising developments that employ aqueous-based routes for the manufacture of high-tech components and specialty products. Such applications include producing metallic and ceramic powders; etching; surface modification by electroplating and electroless plating; manufacturing jewelry and intricate components by electroforming; and producing advanced ceramics, composites, and nanophase materials by sol-gel and biomimetic processing.
Similar content being viewed by others
References
G.A. Warren, “Innovations in Materials Processing: Throw Away the Black Armbands,” J. Met., 40(7) (1988), p. 20.
F.M. Doyle et al., eds., Innovations in Materials Processing using Aqueous, Colloid and Surface Chemistry (Warrendale, PA: TMS, 1989), pp. v–vi.
F.M. Doyle, “Aqueous Processing of Minerals and Materials,” JOM, 41(4) (1989), pp. 51–58.
F.M. Doyle, “Aqueous Processing of Minerals and Materials,” JOM, 42(4) (1990), pp. 52–59.
F.M. Doyle, “Aqueous Processing of Minerals, Metals and Materials,” JOM,43(4) (1991), pp. 43–51.
F.M. Doyle and S. Duyvesteyn, “Aqueous Processing of Minerals, Metals and Materials,” JOM, 45(4) (1993), pp. 46–54.
Metal Finishing Industry Market Survey 1992–1993, Metal Finishing Suppliers Association and National Association of Metal Finishers (March 1994).
M. Murphy, Annual Reviews in February issues of Metal Finishing, 1992,1993,1994.
Surface Cleaning, Finishing, and Coatings, Metals Handbook, vol. 5,9th ed. (Materials Park, OH: ASM, 1982).
S.H. Pawar, M.M. Tonape, and V.N. Shinde, “Pulse Electrodeposition of Y-Ba-Cu Alloyed Films from an Aqueous Bath,” Mater. Chem. Phys., 35 (1993), pp. 86–91.
S.H. Pawar and M.H. Pendse, “Electrodeposition of Dy-Ba-Cu Alloyed Films from Aqueous Bath,” Mater. Res. Bull., 26 (1991), pp. 641–648.
V. Krishan et al., “Electrosynthesis of Thin Films of CdZnSe: Composition Modulation and Bandgap Engineering in the Ternary System,” J. Electrochem. Soc., 139(1) (1992), pp. 23–27.
S.N. Sahu, “Aqueous Electrodeposition of InP Semiconductor Films,” J. Mater. Sci. Lett., 8 (1989), pp. 533–534.
S.N. Sanu, “Structural, Optical and Electrical Properties of First Aqueous Electrodeposited InP Semiconductor Films,” Sol. Energy Mater., 20 (1990), pp. 349–358.
W.D. Fields et al., “ Electroless Nickel Plating, ” Surface Cleaning, Finishing, and Coatings, in ref. 9, pp. 219-243.
N. Feldstein, “Composite Electroless Plating,” Electroless Plating: Fundamentals and Applications, ed. G.O. Mallory and J.B. Hajdu (Orlando, FL: AESF, 1990), pp. 269–287.
J. Zahavi and S. Tamir, “Laser Induced Gold Plating on Nonmetallic Silicon Substrate,” Noble Metals Fabrications and Technology Seminar, ed. L. Gal-Or (Jerusalem, Israel: International Precious Metals Institute, 1985), pp. 169–191.
Kh. Brenner, “Application of Three-Dimensional Components formed by Lithography, Electroforming and Plastic Molding,” Appl. Optics, 32(32) (1993), pp. 6464–6469.
R. Bailey, “Looking Ahead with the Industrial Giant Xerox—An Innovator and Consistent Performer Explores New Uses for Electroforming,” Plat. Surf. Finish., 80 (12) (1993), pp. 26–28.
H.H. Law et al., “Prototype Manufacture of Miniature Nickel/Iron Alloy Magnetic Sleeves for Optical Switching,” Plat. Surf. Finish., 79(8) (1992), pp. 50–54.
J. Song, “Comparison between Precision Roughness Masterspecimens and their Electroformed Replicas,” Precis. Eng., 14(2) (1192), pp. 84–90.
R. Altkorn, “Electroformed Replication of Smooth Mirrors from Sapphire Masters,” Appl. Optics, 31(25) (1992), pp. 5153–5154.
R. Altkorn et al., “Electroformed Replication of Ultra-smooth Mirrors for X-Ray Astronomy,” Proceedings of the International Society for Optical Engineering (SPIE), 1779 (1992), pp. 88–94.
G.A. DiBari, “Electroforming,” Electroplating Engineering Handbook, 4th ed., ed. L.J. Durney (New York: Van Nostrand Reinhold, 1984), pp. 474–190.
J.W. Dini, Electrodeposition—The Materials Science of Coatings and Substrates (Park Ridge, NJ: Noyes, 1993).
M.J. Sole, “Electroforming—a Method for the Future?” Proceedings of World Gold Council International Gold Jewellery Technology, Symposium I: Casting, Gold Technology (11) (Milan, Italy: World Gold Council, Industrial Division, 1993).
D. Tench, “High-Temperature Tensile Properties of Cu-Ag Composites Prepared by Solution Precipitation of Elec-troformed Multilayered Alloys,” J. Mater. Sci., 27(19) (1992), pp. 5286–5290.
MJ. Sole, “Electroforming: Methods, Materials and Merchandise,” JOM, 46(7) (1994), pp. 29–35.
G. Stix, “Micron Machinations,” Sci. Amer., 267(11) (1992), pp. 106–117.
R. Ueda et al., “Principles of Photoetching in the Fabrication of Fine-Pitch Lead Frames,” Met. Finish., 92(1) 1994, pp. 29–31.
E. Peissker, “Production and Properties of Electrolytic Copper Powder,” Int. J. Powder Metall, 20(2) (1984), pp. 87–101.
P.W. Taubenblat, “Production of Copper Powder by Electrolysis,” Powder Metallurgy, Metals Handbook, vol. 7,9th ed. (Materials Park, OH: ASM, 1984), pp. 110–116.
P.K. Samal, “Production of Iron Powder by Electrolysis,” in Ref. 32, pp. 93–96.
O.A. Short and R.V. Weaver, U.S. patent 3,725,035 (1973).
B. Meddings, “Production of Nickel Powder by Hydro-metallurgical Processing,” in Ref. 32, pp. 138–142.
B. Meddings, “Production of Cobalt and Cobalt Alloy Powders,” in Ref. 32, pp. 144–146.
B. Meddings, “Production of Composite Powders,” in Ref. 32, pp. 173–175.
O.N. Collier and S.J. Hackett, U.S. patent 4,274,877 (1981).
M. Blesa and R.J. Candal, “Powder Production for Aqueous Solutions for Ceramics Applications,” Key Eng. Mater., 58 (1991), pp. 107–128.
J. Livage, M. Henry, and J.P. Jolivet, “Inorganic Polymerization in Aqueous Solutions,” Chemical Processing of Ad-vanced Materials, ed. L.L. Hench and J.K. West (New York: Wiley, 1992), pp. 223–237.
M. Ozaki, “Preparation and Properties of Well-Defined Magnetic Particles,” Mater. Res. Bull., 24(12) (1989), pp. 35–40.
Y. Kimishima et al., “Magnetic Study on the Precipitate from the Aqueous Solutions of NiCl2.6H2O and Na2SiO3nH2O,” J. Magn. Magn. Mater., 104-107 (1992), pp. 781–782.
S.R. Sheen et al., “Synthesis and Characterization of High-Tc Y-Ba-Cu-O Superconducting Oxides by Coprecip-itation from Triethylamine-Oxalate Media,” Mater. Lett., 10(11,12) (1991), pp. 489–493.
Y.D. Yao et al., “Aqueous Coprecipitation Synthesis of YBa2Cu3O7-x Superconductors,” J. Mater. Sci. Lett., 12 (1993), pp. 232–233.
F.A. Tourinho, R. Franck, and R. Massart, “Aqueous Ferrofluids Based on Manganese and Cobalt Ferrites,” J. Mater. Sci., 25 (1990), pp. 3249–3254.
G. Guzman et al., “Synthesis of Ferroelectric Perovskites through Aqueous-Solution Techniques,” J. Mater. Sci., 28 (1993), pp. 6510–6515.
B.S. Chiou and J.N. Kuo, “Fabrication and Properties of PLZT Ceramics from Spray-Dried Aqueous Solution,” J. Electron. Mater., 20 (4) (1991), pp. 325–330.
Z.C. Chen, T. A. Ring, and J. Lemaître, “Stabilization and Processing of Aqueous BaTiO3 Suspension with Polyacrylic Acid,” J. Am. Ceram. Soc., 75 (12) (1992), 3201–3208.
M. Deveau et al., “Processing of Wollanstonite-Mullite Composites from Dense Aqueous Suspensions, Part 2” Ceramic Engineering and Science (Columbus, OH: ACerS, 1993), pp. 840–847.
Y. Hirata and T. Osaki, “Rheology and Consolidation of Aqueous Suspensions with Nanometer-Sized BaTi1-x ZrxO3 Powders,” Mater. Lett., 15 (1992), pp. 31–34.
A. Bleier et al., “Effect of Aqueous Processing Conditions on the Microstructure and Transformation Behavior in AL2O3-ZrO2(CeO2) Composites,” J. Am. Ceram. Soc., 75(10) (1992), pp. 2649–2658.
J. Cesarano III and I.A. Aksay, “Processing of Highly Concentrated Aqueous a-Alumina Suspensions Stabilized with Polyelectrolytes,” J. Am. Ceram. Soc., 71(12) (1988), pp. 1062–1067.
A. Bleier, “Secondary Minimum Interactions and Hetero-coagulation Encountered in the Aqueous Processing of Alu-mina-Zirconia Ceramic Composites,” Colloids Surf., 66 (1992), pp. 157–179.
J.W. Nicholson and J.P. Tibaldi, “Formation and Properties of Cements Prepared from Zinc Oxide and Aqueous Solutions of Zinc Nitrate,” J. Mater. Sci., 27 (1992), pp. 2420–2422.
V. DeSapio, “Advanced Structural Ceramics: Challenges to Commercialization,” ChemTech, (11) (1993), pp. 46–51.
N.V. Mandik and G.A. Krulik, “Substitution of Nonhaz-ardous for Hazardous Process Chemicals in the Printed Circuit Industry,” Met. Finish., 90(11) (1992), pp. 49–51.
J. Smith, “In-Line Semi-Aqueous Cleaning of Surface Mount PCBs,” Electron. Packag. Prod. (SuppL) (8) (1992), pp. 56–61.
R.E. Wedin, “How New Solvents Washed Away Bad Press,” Today’s Chemist at Work (September 1993), pp. 10–14.
K. Wolf, “Printed Circuit Board Defluxing: Alternatives toOzone-DepletingSubstances,” Solvent Substitution (Springfield, VA: NTIS, 1990), pp. 127–130.
S.J. Fox, “Alternatives to CFC-Solvent Based Cleaning for PCBs: Which Options are Industry Participants Choosing,” Critical Materials Processes in a Changing World, vol. 6 (Paper presented at the International SAMPE Electronics Conference, 1992), pp. 165–174.
D. Samsami, “Cleaning without CFCs: The Semi-Aque-ous Solutions,” Electron. Packag. Prod. (6) (1991), p. 62.
L. Nudo, “Heirs to the Throne,” Pollut. Eng. (June 1993), pp. 55–58.
H.A. Allcock, “Rational Design and Synthesis of New Polymeric Materials,” Science, 255 (1992), pp. 1106–1112.
F. Aldinger and H.J. Kalz, “The Importance of Chemistry in the Development of High-Perf ormance Ceramics,” Angew. Chemie, Int. Ed. Eng., 26 (5) (1987), pp. 371–381.
D.R. Ulrich, “Prospects for Sol-Gel Processes,” J. Non-Cryst. Sol., 121 (1990), pp. 465–479.
D.R. Ulrich, “Chemical Science’s Impact on Future Glass Research,” Ceram. Bull., 64(11) (1985), pp. 1444–1448.
R. Roy, “Ceramics by the Solution Sol-Gel Route,” Science, 238(12) (1987), pp. 1664–1669.
H. Dislich and P. Hinz, “History and Principles of the Sol-Gel Process, and Some New Multicomponent Oxide Coatings,” J. Non-Cryst. Solids, 48 (1982), pp. 11–16.
B.D. Fabes and D.R. Uhlmann, “Sol-Gel Processing of Glasses and Ceramics,” in Ref. 2, pp. 127–143.
R.W. Jones, Fundamental Principles of Sol-Gel Technology (London: Institute of Metals, 1989).
B.J.J. Zelinski and D.R. Uhlmann, “Gel Technology in Ceramics,” J. Phys. Chem. Solids, 45(10) (1984), pp. 1069–1090.
R.D. Shoup, “Sol-Gel Processes,” Ceramics and Glasses, ASM Engineered Materials Handbook, vol. 4 (Materials Park, OH: ASM, 1987), pp. 445–452.
P.K. Gallagher, “Chemical Synthesis,” in Ref. 72, pp. 52–64.
L.C. Klein, “Sol-Gel Process,” in Ref. 72, pp. 209–214.
J. Zaraycki, M. Prassas, and J. Phalippou, “Synthesis of Glasses from Gels: The Problem of Monolithic Gels,” J. Mater. Sci., 17 (1982), pp. 3371–3379.
A.C. Pierre, “Sol-Gel Processing of Ceramic Powders,” Ceram. Bull., 70(8) (1991), pp. 1281–1288.
D.W. Johnson, Jr., “Sol-Gel Processing of Ceramics and Glass,” Am. Ceram. Soc. Bull., 64(12) (1985), pp. 1597–1602.
H.G. Sowman, “A New Era in Ceramic Fibers via Sol-Gel Technology,” Ceram. Bull., 76(12) (1988), pp. 1911–1916.
S. Sakka, “Sol-Gel Synthesis of Glasses: Present and Future,” Am. Ceram. Soc. Bull., 64(11) (1985), pp. 1463–1466.
J.D. MacKenzie, R. Xu, and X. Yuhuan, “Ultrastructure Processing of Thin Crystalline Films,” in Ref. 40, pp. 365–378.
C.J. Brinker et al., “Sol-Gel Thin-Film Formation,” in Ref. 40, pp. 395–414.
G.R. Lee and J.A. Crayston, “Sol-Gel Processing of Transition Metal Alkoxides for Electronics,” Adv. Mater., 5(6) (1993), pp. 434–442.
J. Covino and A.C. Finlinson, “Sol-Gel-Derived Coatings for Electrical and Optical Applications,” in Ref. 40, pp. 457–466.
L.M. Sheppard, “Advances in Processing of Ferroelectric Thin Films,” Ceram. Bull., 71(1) (1992), pp. 85–95.
B.M. Melnick et al., “Process Optimization and Characterization of Device Worthy Sol-Gel Based PZT for Ferroelectric Memories,” Ferroelectrics, 112 (1990), pp. 329–351.
Anon., “Forecast ’92: Trends in Materials Processing,” Adv. Mater. Proc. (1) (1992), pp. 56–57.
R. Roy, “Solution-Sol-Gel Technology and Science: Past, Present and Future,” in Ref. 40, pp. 1023–1033.
P.D. Calvert, “Biomimetic Mineralization: Processes and Prospects,” Mater. Sci. Eng. C, 1(2) (1994), pp. 69–74.
C.-W. Li and B.E. Volcani, “Solidification in Diatom Walls,” Phil Trans. Roy. Soc. London B, 304 (1984), pp. 519–528.
F.Z. Cui et al., “Anisotropie Indentation Morphology and Hardness of Natural Ivory,” Mater. Sci. Eng. C, 1(3) (1994), in press.
J.E. Mark and P.D. Calvert, “Biomimetic, Hybrid and In-Situ Composites,” Mater. Sci. Eng. C, 1(3) (1994), in press.
A.H. Heuer et al., “Innovative Materials Processing Strategies: A Biomimetic Approach,” Science, 255 (1992), pp. 1098–1105.
C. Viney, “Processing and Microstructural Control: Les-sons from Natural Materials,” Mater. Sci. Eng. R, 10 (1993) pp. 187–236.
S.A. Wainwright et al., Mechanical Design in Organisms (London, U.K.: Arnold, 1976).
P.D. Calvert, “In Situ Composites: Polymers,” Encyclopedia of Advanced Materials, ed. D. Bloor et al. (Oxford, U.K.: Pergamon Press, 1988).
J.F.V. Vincent, Structural Biomaterials (London: Macmillan, 1982).
S. Mann, R.B. Frankel, and R.P. Blakemore, “Structure, Morphology and Crystal Growth of Bacterial Magnetite,” Nature, 310 (1985), pp. 405–407.
J. Burdon and P. Calvert, “Orientation in Biomimetic Polymer/Mineral Composites,” Mater. Res. Soc. Symp. (Hierarchically Structured Materials), vol. 255, ed. I. Aksay et al. (Pittsburgh, PA: Materials Research Society, 1992), pp. 375–383.
B.L. Zhou, “The Biomimetic Study of Composite Mate-rials,” JOM, 46(2) (1994), pp. 57–62.
B.J. Tarasevich et al, “Synthesis of Ceramic Ultrastructures Utilizing Biological Processes,” in Ref. 40, pp. 529–542.
R.P. Feynman, “There’s Plenty of Room at the Bottom: An Invitation to Enter a New Field of Physics” (Paper presented at the Annual Meeting of the American Physical Society, 29 December 1959). Reprinted in Nanotechnology Research and Perspectives, ed. B.C. Crandall and J. Lewis ( Cambridge, MA: MIT Press, 1992), pp. 347–363.
K.E. Drexler, Engines of Creation (New York: Doubleday, 1986).
K.E. Drexler, Nanosystems: Molecular Machinery, Manufacturing and Computation (New York: Wiley, 1992).
D.J. Whitehouse and K. Kawata, eds., Nanotechnology (New York: Adam Hilger, 1990).
J.W. Gardner and H.T. Hingle, eds., From Instrumentation to Nanotechnology (Reading, UK: Gordon and Breach, 1991).
J. Morse, “The Next Industrial Revolution: Molecular Nanotechnology,” Colo. Sch. Mines Quart. Rev., 93(3) (1993), pp. 1–5.
F. Hapgood, “The Really Little Engines that Might,” Technol. Rev., 96(2) (1993), pp. 31–36.
N.C. MacDonald, “Nanomechanisms: Materials, Structures and Devices” (Paper presented at the 123rd TMS Annual Meeting, San Francisco, CA, 1994).
F.A. Buot, “Mesoscopic Physics and Nanoelectronics: Nanoscience and Nanotechnology,” Phys. Rep., 234(2 & 3) (1993), pp. 73–174.
K.E. Drexler, “Large-Scale Atomic Precision” (Paper presented at 123rd TMS Annual Meeting, San Francisco, CA, 1994).
B.C. Crandall and J. Lewis, eds., Nanotechnology Research and Perspectives (Cambridge, MA: MIT Press, 1992).
M. Sarikaya and I.A. Aksay, “Synthetic and Biological Nanocomposites,” in Ref. 40, pp. 543–555.
S. Mann, “Crystallization at Inorganic/Organic Interfaces—Biominerals and Biomimetic Synthesis,” Science, 261 (5126) (1993), pp. 1286–1292.
L.L. Clements, “A Materials Engineer looks at Nanotechnology: The Science and the Science Fiction,” Submitted to Mater. Sci. Eng. C (1993).
G. Zhang et al., “Biological Synthesis of Monodisperse Derivatives of Poly(a,L-Glutamic Acid): Model Rodlike Polymers,” Macromolec, 25 (1992), pp. 3601–3603.
K.P. McGrath et al., “Genetically Directed Syntheses of New Polymeric Materials: Expression of Artificial Genes Encoding Proteins with Repeating-(AlaGly)3-ProGluGly-Elements,” J. Am. Chem. Soc., 114 (1992), pp. 727–733.
G.-M. Chow, M.A. Markowitz, and A. Singh, “Synthe-sizing Submicrometer and Nanoscale Particles via Self-Assembled Molecular Membranes,” JOM, 45(11) (1993), pp. 62–65.
G.M. Chow et al., “Metallic and Ceramic Nanostructures via Bio/Molecular Routes” (Paper presented at the 123rd TMS Annual Meeting, San Francisco, CA, 1994).
M.-P. Pileni, “Water in Oil Colloidal Droplets Used as Microreactors,” Adv. Colloid Interf. Sci., 46 (1993), pp. 139–163.
Lisiecki and M.P. Pileni, “Synthesis of Copper Metallic Clusters using Reverse Micelles,” J. Am. Chem. Soc., 115 (1993), pp. 3887–3896.
A.J.I. Ward and S.E. Friberg, “Preparing Narrow Size Distribution Particles from Amphiphilic Association Structures,” Mater. Res. Bull. (12) (1989), pp. 41–46.
S.E. Friberg and C.C. Yang, “Silica Glass from Water/Oil Microemulsions,” in Ref. 2, pp. 181–191.
D.O. Shah et al., “Formation of Ultrafine Particles using Water/Oil Microemulsions for Silver Halide, Iron Oxide and Superconducting Oxide,” in Ref. 2, pp. 193–211.
A. Henglein, “Small-Particle Research: Physicochemical Properties of Extremely Small Colloidal Metal and Semicon-ductor Particles,” Chem. Rev., 89 (1989), pp. 1861–1871.
M.G. Bawendi, M.L. Stiegerwald, and L.E. Brus, “The Quantum Mechanics of Larger Semiconductor Clusters (“Quantum Dots”),” Ann. Rev. Phys. Chem., 41 (1990), pp. 477–496.
M.L. Steigerwald and L.E. Brus, “Semiconductor Crystallites: A Class of Large Molecules,” Acc. Chem. Res., 23 (1990), pp. 183–188.
Y. Wang, “Nonlinear Optical Properties of Nanometer-Sized Semiconductor Clusters,“ Ace Chem. Res., 24 (1991), pp. 133–139.
R.D. Shull and J.J. Ritter, “Chemically Prepared Mag-netic Nanocomposites” (Paper presented at the 123rd TMS Annual Meeting, San Francisco, CA, 1994).
R.T.C. Choo and J.M. Toguri, “Mass Transfer Analysis in the Pulse Plating of Nickel Nanocrystals” (Paper presented at the 123rd TMS Annual Meeting, San Francisco, CA, 1994).
T.L. Trentler et al., “Sonochemical Synthesis of Nanom-eter-Sized Particles of Molybdenum Suicides” (Paper presented at the 123rd TMS Annual Meeting, San Francisco, CA, 1994).
R.D. Shull, “Nanometer-Scale Materials and Technol-ogy,” JOM, 45(11) (1993), pp. 60–61.
X.K. Zhao, L.D. McCormick, and J.H. Fendler, “Preparation-Dependent Rectification Behavior of Lead Sulfide Particulate Films,” Adv. Mater., 4(2) (1992), pp. 93–94.
T. Douglas and S. Mann, “Oriented Nucleation of Gypsum under Compressed Langmuir Monolayers,” Mater. Sci. Eng. C, 1(3) (1994), in press.
L.J. Sealock et al., “Chemical Processing in High-Pressure Aqueous Environments. 1. Historical Perspective and Continuing Developments,” Ind. Eng. Chem. Res., 32 (1993), pp. 1535–1541.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mooiman, M.B., Sole, K.C. Aqueous processing in materials science and engineering. JOM 46, 18–28 (1994). https://doi.org/10.1007/BF03220714
Issue Date:
DOI: https://doi.org/10.1007/BF03220714