Summary
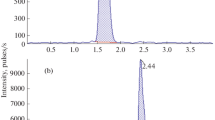
A rapid, simple and sensitive high-performance liquid chromatography (HPLC) method was established for the quantification of nifuratel in human plasma and applied to a study of its pharmacokinetics. A test and a reference formulation were investigated and compared, and the study group consisted of 24 healthy male volunteers. The analytical technique was based on a single extraction of the drug from the plasma with chloroform, using ornidazole as internal standard (IS). The chromatographic system consisted of a 5-μm 4.6 mmX250 mm C18 analytical column and the mobile phase consisted of methanol and purified water (45∶55, v/v). Nifuratel and ornidazole concentrations were detected by ultraviolet (UV) absorbance at a wavelength of 254 nm. The lower limit of detection and quantification was 0.5 ng·ml−1, and the calibration curves were linear over a concentration range of 0.5–160 ng·ml1 nifuratel in the plasma. The results showed that the area under the plasma concentration-time curve (AUC), time to maximum observed plasma concentration (Tmax), maximum concentration reached in the concentration profile (Cmax), and elimination half-life (t1/2) between the test tablets and the reference tablets demonstrated no significant difference (P>0.05). The relative bioavailability amounted to 103.13% ± 8.73%.
Similar content being viewed by others
References
Karagozov I., Shopova E., Poriazov K., Abaliev I., Kozovski I., Ianeva R., Drenska S., Vasileva P., Bozhinova S., Popov I., Stoikov S. (1999): A multicenter study of the antimicrobial effect of Macmiror and Macmiror complex in the treatment of vaginal infections. Akush Ginekol., 38(3): 61–62.
Gruneberg R.N., Leakey A. (1976): Treatment of candidal urinary tract infection with nifuratel. Br. Med. J., 2(6041): 908–910.
Guinebault P.R., Broquaire M., Braithwaite R.A. (1981): Determination of nifuroxazide in biological fluids by automated high-performance liquid chromatography with large-volume injection. J. Chromatogr., 16(204): 329–333.
Liu J.A., Wang M.X., Meng Y. (2003): Research on determination of the content and the related substances of nifuratel by HPLC. Chin. J. Pharm. Anal., 23(1):28–31.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, XL., Li, BY., Ni, MY. et al. An improved HPLC method for determination of nifuratel in human plasma and its application to pharmacokinetics studies. Eur. J. Drug Metabol. Pharmacokinet. 32, 69–73 (2007). https://doi.org/10.1007/BF03190994
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03190994