Abstract
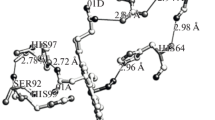
The crystal structure of sperm whale myoglobin (Mb):azide complex has been determined and refined at 1.8 Å resolution. The ligand is coordinated to the heme iron, and deeply buried in the inner part of the distal site. The structural organization of this complex differs substantially from that observed inA. limacina Mb: azide complex, in which the iron coordinated ligand is directed towards the outer solvent region of the protein. In the case of sperm whale Mb the bound ligand is stabilized by a hydrogen bond to residue HisE7; in the case ofA. limacina Mb the ligand is directly hydrogen bonded to ArgE10. The structures of the complexes described underline different mechanisms of ligand recognition and stabilization in the two Mb’s, and allow a rational interpretation of the different kinetic and thermodynamic properties observed in globins lacking a polar residue at the distal E7 site.
Riassunto
La struttura cristallina del complesso formato dalla mioglobina (Mb) di capodoglio e dall’azide è stata determinata a 1.8 Å di risoluzione. Il legante risulta coordinate al ferro eminico, e profondamente sepolto nella parte più interna del sito distale. L’organizzazione strutturale di questo complesso differisce da quella dell’analogo addotto formato dalla Mb diA. limacina e dall’azide in cui il legante è orientato verso l’esterno del sito distale della proteina. Nel caso della Mb di capodoglio il legante è stabilizzato da un legame idrogeno con il residuo HisE7; nel caso della Mb di Alimacina, il legante forma un legame idrogeno con il residuo ArgE10. Le strutture dei complessi molecolari descritti indicano l’esistenza di due diversi meccanismi di riconoscimento e stabilizzazione del legante nelle due Mb, e permettono di interpretare le diverse proprietà termodinamiche e cinetiche osservate nelle globine prive di un residuo polare al sito distale E7.
Similar content being viewed by others
References
M. F. Perutz,Myoglobin and Haemoglobin: Role of Distal Residues in Reactions with Haem Ligands. Trends Biochem. Sci., 14, 1989, 42–44.
A. F. Riggs,Hemoglobins. Curr. Opinion Struct. Biol., 1, 1991, 915–921.
R. E. Dickerson -I. Geis,Haemoglobin Structure Function and Pathology. The Benjamin/Cummings Publishing Co., Menlo Park, CA., 1983.
E. Antonini -M. Brunori,Hemoglobin and Myoglobin in their Reactions with Ligands. Elsevier North-Holland Publishing Co., Amsterdam 1971.
T. Takano,Structure of Myoglobin Refined at 2.0 Å Resolution. Crystallographic Refinement of Metmyoglobin from Sperm Whale. J. Mol. Biol., 110, 1977, 537–568.
S. E. V. Phillips,Structure and Refinement of Oxymyoglobin at 1.4 Å Resolution. J. Mol. Biol., 142, 1980, 531–554.
A. Mattevi -G. Gatti -A. Coda -M. Rizzi -P. Ascenzi -M. Brunori -M. Bolognesi,Binding mode of azide to ferric Aplysia limacinamyoglobin: crystallographic analysis at 1.9 Å resolution. J. Mol. Recognition, 4, 1991, 1–6.
M. Bolognesi -A. Coda -F. Frigerio -G. Gatti -P. Ascenzi -M. Brunori,X-ray Crystal Structure of the Fluoride Derivative of Aplysia limacinaFerric Myoglobin at 2.0 A Resolution. Stabilization of the Fluoride Ion by Hydrogen Bonding to Arg66 (E10). J. Mol. Biol., 213, 1990, 621–625.
M. Bolognesi -S. Onestt -G. Gatti -A. Coda -P. Ascenzi -M. Brunori, Aplysia limacinaMyoglobin. Crystallographic Analysis at 1.6 Å Resolution. J. Mol. Biol., 205, 1989, 529–544.
L. Stryer -J. C. Kendrew -H. C. Watson,The Mode of Attachment of the Azide Ion to Sperm Whale Met Myoglobin. J. Mol. Biol., 8, 1964, 96–104.
M. Bolognesi -E. Cannillo -P. Ascenzi -G. M. Giacometti -A. Merli -M. Brunori,Reactivity of Ferric Aplysia and Sperm Whale Myoglobins toward Imidazole. X-ray and Rinding Study. J. Mol. Biol., 150, 1982, 305–315.
F. C. Bernstein -T. F. Koetzle -G. J. B. Williams -E. F. Jr. Meyer -M. D. Brice -J. R. Rodgers -O. Kennard -T. Shimanouchi -M. Tasumi,The Protein Data Bank. A Computer-based Archival File for Macromolecular Structures. J. Mol. Biol., 112, 1977, 535–542.
D. E. Tronrud -L. F. Ten Eyck -B. W. Matthews,An Efficient General- Purpose Least-Squares Refinement Program for Macromolecular Structures. Acta Crystallogr., sect. A., 43, 1987, 489–501.
T. A. Jones,A Graphics Model Building and Refinement System for Macromolecules. J. Appl. Crystallogr., 11, 1978, 268–272.
B. Seamonds -R. E. Forster -G. Philip,Physicochemical Properties of the Hemoglobins from Common Bloodworm Glycera dibranchiata. J. Biol. Chem., 246, 1971, 5391–5397.
G. M. Giacometti -A. Da Ros -E. Antonini -M. Brunori,Equilibrium and Kinetics of the Reaction of Aplysia Myoglobin with Azide. Biochemistry, 14, 1975, 1584–1588.
M. Bolognesi -F. Frigerio -C. Lionetti -M. Rizzi -P. Ascenzi-M. Brunori, Aplysia limacinaMyoglobin: Molecular Bases for Ligand Binding. In:S. N. Vinogradov -O. H. Kapp (eds.),Structure and Function of Invertebrate Oxygen Carriers. Springer-Verlag, New York 1991, 161–170.
C. Lionetti -M. G. Guanziroli -F. Frigerio -P. Ascenzi -M. Bolognesi,X-ray Crystal Structure of the Ferric Sperm Whale Myoglobin:Imidazole Complex at 2.0 Å Resolution. J. Mol. Biol., 217, 1991, 409–412.
W. Steigemann -E. Weber,Structure of Erythrocruorin in Different Ligand States Refined at 1.4 Å Resolution. J. Mol. Biol., 127, 1979, 309–338.
B. Benko -S. Maricic,Haem Accessibility in Monomeric Hemoglobins of Glycera dibranchiataand Petronuyzon marinus,a Proton Magnetic Resonance Study. Croat. Chem. Acta, 51, 1978, 369–377.
F. Cutruzzolà -C. Travaglini Allocatelli -P. Ascenzi -M. Bolognesi -S. G. Sugar -M. Brunori,Control and Recognition of Anionic Ligands in Myoglobin. FEBS Lett., 282, 1991, 281–284.
Author information
Authors and Affiliations
Additional information
Nella seduta del 9 maggio 1992.
Residues of sperm whale andA. limacina Mb have been identified by their topological positions as defined by Perutz [1, 3]. The eight helices in the globin fold are identified by letters A,B,...H. The symbols AB, BC, ..., GH, indicate the nonhelical regions connecting helices A and B, B and C, ..., G and H. Numbering and nomenclature of the heme group are those adopted by Takano [5]. Water molecules (W) have been numbered sequentially, starting from 150. Abbreviations used: myoglobin, Mb; monomeric hemoglobin, Hb; monomeric erythrocruorin, Ery.
Rights and permissions
About this article
Cite this article
Rizzi, M., Ascenzi, P., Coda, A. et al. Molecular bases for heme:ligand recognition in sperm whale (Physeter Catodon) andAplysia limacine myoglobin. Rend. Fis. Acc. Lincei 4, 65–73 (1993). https://doi.org/10.1007/BF03001186
Issue Date:
DOI: https://doi.org/10.1007/BF03001186