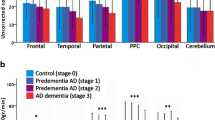
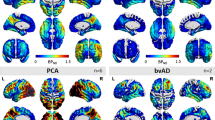
Abstract
In this review I summarize observations of PET and SPECT studies about cerebral blood flow and metabolic abnormalities in Alzheimer’s disease. In very early AD flow or metabolism reduces first in the posterior cingulate gyrus and precuneus. This reduction may arise from functional deafferentation caused by primary neural degeneration in the remote area of the entorhinal cortex that is the first to be pathologically affected in AD. Then medial temporal structures and parieto-temporal association cortex show flow or metabolic reduction as disease processes. The reason why flow or metabolism in medial temporal structures shows delay in starting to reduce in spite of the earliest pathological affection remains to be elucidated. It is likely that anterior cingulate gyrus is functionally involved, since attention is the first non-memory domain to be affected, before deficits in language and visuospatial functions. However few reports have described involvement in the anterior cingulate gyrus. Relationship between cerebral blood flow or metabolism and apolipoprotein E genotype has been investigated. Especially, theAPOE ε4 allele has been reported to increase risk and to lower onset age as a function of the inherited dose of the ε4 allele. Reduction of flow or metabolism in the posterior cingulate gyrus and precuneus has been reported even in presymptomatic nondemented subjects who were cognitively normal and had at least a single ε4 allele. On the contrary the relation of ε4 allele to the progression rate of AD has been controversial from neuroimaging approaches. PET and SPECT imaging has become to be quite useful for assessing therapeutical effects of newly introduced treatment for AD. Recent investigations observed significant regional flow increase after donepezil hydrochloride treatment. Most of these observations have been made by applying computer assisted analysis of three-dimensional stereotactic surface projection or statistical parametric mapping instead of a conventional regions of interest technique.
Similar content being viewed by others
References
Minoshima S, Berger KL, Lee KS, Mintun MA. An automated method for rotational correction and centering of three-dimensional functional brain images.J Nucl Med 1992; 33: 1579–1585.
Minoshima S, Koeppe RA, Mintun MA, et al. Automated detection of the intercommissural (AC-PC) line for stereotactic localization of functional brain images.J Nucl Med 1993; 34: 322–329.
Minoshima S, Koeppe RA, Frey KA, Kuhl DE. Anatomical standardization: linear scaling and nonlinear warping of functional brain images.J Nucl Med 1993; 35: 1528–1537.
Frith CD, Friston KJ, Ashburner J, et al. Principles and methods. InHuman Brain Function, Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC (eds), 1st ed, San Diego; Academic Press, 1997: 3–159.
Emilinen G, Beyreuther K, Masters CL, Maloteaux JM, Prospects for pharmacological intervention in Alzheimer disease.Arch Neurol 2000; 57: 454–459.
Friedland RP, Budinger TF, Ganz E, et al. Regional cerebral metabolic alterations in dementia of the Alzheimer type: positron emission tomography with [18F]fluorodeoxyglucose.J Comput Assist Tomogr 1983; 7: 590–598.
Foster NL, Chase TN, Fedio P, Psztonas NJ, Brooks RA, Di Chiro G. Alzheimer’s disease: focal cortical changes shown by positron emission tomography.Neurology 1983; 33: 961–965.
Kuhl DE. Imaging local brain function with emission computed tomography.Radiology 1984; 150: 625–631.
Foster NL, Chase TN, Mansi L, et al. Cortical abnormalities in Alzheimer’s disease.Ann Neurol 1984; 16: 649–654.
Kuhl DE, Metter EJ, Riege WH. Patterns of cerebral glucose utilization in depression, multiple infarct dementia, and Alzheimer’s disease.Res Pub Assoc Res Nerv Ment Dis 1985; 63: 211–226.
Cutler NR, Haxby JV, Duara R, et al. Clinical history, brain metabolism, and neuropsychological function in Alzheimer’s disease.Ann Neurol 1985; 18: 298–309.
Friedland RP, Budinger TF, Koss E, Ober BA. Alzheimer’s disease: anterior-posterior and lateral hemispheric alterations in cortical glucose utilization.Neurosci Lett 1985; 53: 235–240.
Duara R, Grady C, Haxby JV, et al. PET in Alzheimer’s disease.Neurology 1986; 36: 879–887.
McGeer PL, Kamo H, Harrop R, et al. Positron emission tomography in patients with clinically diagnosed Alzheimer’s disease.Can Med Assoc J Neurol Neurosurg Psychiatry 1986; 134: 597–607.
Polinsky RJ, Noble H, Di Chiro G, Nee LE, Feldman RG, Brown RT. Dominantly inherited Alzheimer’s disease: cerebral glucose metabolism.J Neurol Neurosurg Psychiatry 1987; 50: 752–757.
Johnson KA, Holman BL, Mueller SP, et al. Single photon emission computed tomography in Alzheimer’s disease: abnormal iofetamine I 123 uptake reflects dementia severity.Arch Neurol 1988; 45: 392–396.
Herholz K, Adams R, Kessler J, Szelies B, Grond M, Heiss WD. Criteria for the diagnosis of Alzheimer’s disease with PET.Dementia 1990; 1: 156–164.
Heiss WD, Szelies B, Kessler J, Herholz K. Abnormalities of energy metabolism in Alzheimer’s disease studies with PET.Ann NY Acad Sci 1991; 640: 65–71.
Guze BH, Hoffman JM, Baxter LR Jr, Mazziota JC, Phelps ME. Functional brain imaging and Alzheimer-type dementia.Alzheimer Dis Assoc Disord 1991; 5: 215–230.
Nyback H, Nyman H, Blomqvist G, Sjorgren I, Stone-Elander S. Brain metabolism in Alzheimer’s dementia: studies of11C-deoxyglucose accumulation, CSF monoamine metabolites and neuropsychological test performance in patients and healthy subjects.J Neurol Neurosurg Psychiatry 1991; 54: 672–678.
Mielke R, Herholz K, Grond M, Kessler J, Heiss WD. Differences of regional cerebral glucose metabolism between presenile and senile dementia of Alzheimer type.Neurobiol Aging 1992; 13: 93–98.
Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET.J Nucl Med 1995; 36: 1238–1248.
Cutler NR, Duara R, Creasey H, et al. NIH Conference. Brain imaging: aging and dementia.Ann Intern Med 1984; 101: 355–369.
Koss E, Friedland RP, Ober BA, Jagust WJ. Differences in lateral hemispheric asymmetries of glucose utilization between early- and late-onset Alzheimer-type dementia.Am J Psychiatry 1985; 142: 638–640.
McGeer EG, Peppard RP, McGeer PL, et al. Fluorine-18-fluorodeoxyglucose positron emission tomography studies in presumed Alzhemmer cases, including 13 serial scans.Can J Neurol Sci 1990; 17: 1–11.
Kumar A, Scapiro MB, Haxby JV, Grady CL, Friedland RP. Cerebral metabolic and cognitive studies in dementia with frontal lobe behavioral features.J Psychiatr Res 1990; 24: 97–109.
Haxby JV, Grady CL, Koss E, et al. Longitudinal study of cerebral metabolic asymmetries and associated neuropsychological patterns in early dementia of the Alzheimer type.Arch Neurol 1990; 47: 753–760.
Smith GS, Leon ML, George AE, et al. Topography of cross-sectional and longitudinal glucose metabolic deficits in Alzheimer’s disease. Pathophysiologic implications.Arch Neurol 1992; 49: 1142–1150.
Brown DRP, Hunter R, Wyper DJ, et al. Longitudinal changes in cognitive function and regional cerebral function in Alzheimer’s disease: a SPECT blood flow study.J Psychiatr Res 1996; 30: 109–126.
Lehtovirta M, Kuikka J, Helisalmi S, et al. Longitudinal SPECT study in Alzheimer’s disease: relation to apolipoprotein E polymorphism.J Neurol Neurosurg Psychiatry 1998; 64: 742–746.
Brun A, Gustafson L. Distribution of cerebral degeneration in Alzheimer’s disease. A clinico-pathological study.Arch Psychiat Nervenkr 1976; 223: 15–33.
Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation.Science 1984; 225: 1168–1170.
Braak H, Braak E. Neuropathological staging of Alzheimer-related changes.Acta Neuropathologica 1991; 82: 239–256.
Braak H, Brank E. Staging of Alzheimer’s disease-related neurofibrillary changes.Neurobiol Aging 1995; 16: 271–284.
Gomez-Isla T, Price TL, McKeel DW, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease.J Neuroscience 1996; 16: 4491–4500.
Jack CR, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease.Neurology 1997; 49: 786–794.
Bobinski M, Leon MJ, Convit A, et al. MRI of entorhinal cortex in mild Alzheimer’s disease.Lancet 1999; 353: 38–40.
Huff FJ, Becker JT, Bell SH, Nebes RD, Holland AL, Boller F. Cognitive deficits and clinical diagnosis of Alzheimer’s disease.Neurology 1987; 37: 1119–1124.
Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of demential in Alzheimer’s disease: use of the neuropsychological measures developed for the consortium to establish a registry for Alzheimer’s disease.Arch Neurol 1992; 49: 448–452.
Pearlson GD, Harris GJ, Powers RE, et al. Quantitative changes in mesial temporal volume, regional cerebral blood flow, and cognition in Alzheimer’s disease.Arch Gen Psychiatry 1992; 49: 402–408.
Ohnishi T, Hoshi H, Nagamachi S, et al. High-resolution SPECT to assess hippocampal perfusion in neuropsychiatric diseases.J Nucl Med 1995; 36: 1163–1169.
Julin P, Lindqvist J, Svensson L, Slomka P, Wahlund LO. MRI-guided SPECT measurements of medial temporal lobe blood flow in Alzhemer’s disease.J Nucl Med 1997; 38: 914–919.
Rodriguez G, Nobili F, Copello F, et al.99mTc-HMPAO regional cerebral blood flow and quantitative electroencephalography in Alzheimer’s disease: a correlative study.J Nucl Med 1999; 40: 522–529.
Kogure D, Matsuda H, Ohnishi T, et al. Longitudinal evaluation of early Alzheimer’s disease using brain perfusion SPECT.J Nucl Med 2000; 41: 1155–1162.
Kitayama N, Kogure D, Ohnishi T, et al. MRI-based volumetry of hippocampal gray-matter, and SPECT measurements of hippocampal blood flow for the diagnosis of Alzheimer’s disease: comparison with Statistical Parametric Mapping.Brain Science and Mental Disorders 1999; 10: 299–306.
Ishii K, Kitagaki H, Kono M, Mori E. Decreased medial temporal oxygen metabolism in Alzheimer’s disease shown by PET.J Nucl Med 1996; 37: 1159–1165.
Ishii K, Sasaki M, Yamaji S, Sakamoto S, Kitagaki H, Mori E. Paradoxical hippocampus perfusion in mild-to-moderate Alzheimer’s disease.J Nucl Med 1998; 39: 293–298.
Dhillon HS, Dose JM, Scheff SW, Prasad MR. Time course of changes in lactate and free fatty acids after experimental brain injury and relationship to morphologic damage.Exp Neurol 1997; 146: 240–249.
Minoshima S, Foster NL, Kuhl DE. Posterior cingulate cortex in Alzheimer’s disease.Lancet 1994; 344: 895.
Minoshima S, Giordani B, Berent S, et al. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease.Ann Neurol 1997; 42: 85–94.
Johnson KA, Jones BL, Holman JA, et al. Preclinical prediction of Alzheimer’s disease using SPECT.Neurology 1998; 50: 1563–1571.
Ibanez V, Pietrini P, Alexandar GE, et al. Regional glucose metabolic abnormalities are not the result of atrophy in Alzheimer’s disease.Neurology 1998; 50: 1585–1593.
Small GW, Mazziotta JC, Collins MT, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease.JAMA 1995; 273: 942–947.
Small GW, Ercoli LM, Silverman DH, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease.Proc Natl Acad Sci USA 2000; 97: 6037–6042.
Mazziota JC, Phelps ME. Principles and Applications for the Brain and Heat. InPositron Emission Tomography and Autoradiography, Phelps ME, Mazziota JC, Schelbert H (eds), New York; Raven Press, 1986: 493–579.
Meguro K, Blaizot X, Kondoh Y, Le Mestric C, Baron JC, Chavoix C. Neocortical and hippocampal glucose hypometabolism following neurotoxic lesions of the entorhinal and perirhinal cortices in the non-human primate as shown by PET. Implications for Alzheimer’s disease.Brain 1999; 122: 1519–1531.
Desgranges B, Baron JC, de la Sayette V, et al. The neural substrates of memory systems impairment in Alzheimer’s disease. A PET study of resting brain glucose utilization.Brain 1998; 121: 611–631.
Fletcher PC, Frith CD, Grasby PM, Shallice T, Frackwiak RSJ, Dolan RJ. Brain system for encoding and retrieval of auditory-verbal memory.Brain 1995; 118: 401–416.
Rudge P, Warrington EK. Selective impairment of memory and visual perception in splenial tumours.Brain 1991; 114: 349–360.
Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia.Brain 1987; 110: 1631–1646.
Papez JW. A proposed mechanism of emotion.Arch Neurol Psychiatry 1937; 38: 725–743.
Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer’s disease. A critical review.Brain 1999; 122: 383–404.
Rizzo M, Anderson SW, Dawson J, Myers R, Ball K. Visual attention impairments in Alzheimer’s disease.Neurology 2000; 54: 1954–1959.
Iidaka T, Anderson ND, Kapur S, Cabeza R, Craok FI. The effect of divided attention on encoding and retrieval in episodic memory revealed by positron emission tomography.J Cogn Neurosci 2000; 12: 267–280.
Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Pertersen SE. Selective and divided attention during Visual Discrimination of shape, color, and speed: functional anatomy by positron emission tomography.J Neurosci 1991; 11: 2383–2402.
Madden DJ, Turkington TG, Provenzale JM, Hawk TC, Hoffman JM, Coleman RE. Selective and divided visual attention: Age-Related changes in regional cerebral blood flow measured by H2 15O PET.Hum Brain Map 1997; 5: 389–409.
Benson DF, Kuhl DE, Hawkins RA, Phelps ME, Cummings JL, Tsai SY. The fluorodeoxyglucose18F scan in Alzheimer’s disease and multi-infarct dementia.Arch Neurol 1983; 40: 711–714.
Minoshima S, Frey KA, Foster NL, Kuhl DE. Preserved pontine glucose metabolism in Alzheimer disease: a reference region for functional brain image (PET) analysis.J Comput Assist Tomogr 1995; 19: 541–547.
Ishii K, Sasaki M, Kitagaki H, et al. Reduction of cerebellar glucose metabolism in advanced Alzheimer’s disease.J Nucl Med 1997; 38: 925–928.
Weisgraber KH. Apolipoprotein E: Structure-function relationship.Adv Protein Chem 1994; 45: 249–302.
Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families.Science 1993; 261: 921–923.
Yamagata Z, Asada T, Kinoshita A, et al. Distribution of apolipoprotein E gene polymorphisms in Japanese patients with Alzheimer’s disease and in Japanese centenarians.Hum Hered 1996; 47: 22–26.
Nagy Z, Esiri MM, Jobst KA, et al. Influence of the Apolipoprotein E genotype on amyloid deposition and neurofibrillary tangle formation in Alzheimer’s disease.Neuroscience 1995; 69: 757–761.
Polvikoski T, Sulkava R, Haltia M, et al. Apolipoprotein E, dementia, and cortical deposition of β-amyloid protein.N Engl J Med 1995; 333: 1242–1247.
Bales KR, Verina T, Dodel RC, et al. Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition.Nature Genet 1997; 17: 263–264.
Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E.N Engl J Med 1996; 334: 752–758.
Craft S, Teri L, Edland SD, Kukull WA, et al. Accelerated decline in apolipoprotein E-epsilon4 homozygotes with Alzheimer’s disease.Neurology 1998; 51: 149–153.
Growdon JH, Locascio JJ, Corkin S, et al. Apolipoprotein E genotype does not influence rates of cognitive decline in Alzheimer’s disease.Neurology 1996; 47: 444–448.
Gomez-Isla T, West HL, Rebeck GW, et al. Clinical and pathological correlates of apolipoprotein E epsilon 4 in Alzheimer’s disease.Ann Neurol 1996; 39: 62–70.
Slooter AJ, Houwing-Duistermaat JJ, van Harskamp F, et al. Apolipoprotein E genotype and progression of Alzheimer’s disease: the Rotterdam Study.J Neurol 1999; 246: 304–308.
Corder EH, Jelic V, Basun H, et al. No difference in cerebral glucose metabolism in patients with Alzheimer’s disease and differing apolipoprotein E genotypes.Arch Neurol 1997; 54: 273–277.
Hirono N, Mori E, Yasuda M, Ishii K, et al. Lacl of association of apolipoprotein E epsilon4 allele dose with cerebral glucose metabolism in Alzheimer disease.Alzheimer Dis Assoc Disord 1998; 12: 362–367.
Frisoni GB, Govoni S, Geroldi C, et al. Gene dose of the epsilon 4 allele of apolipoprotein E and disease progression in sporadic late-onset Alzheimer’s disease.Ann Neurol 1995; 37: 596–604.
van Dyck CH, Gelemter J, MacAvoy MG, et al. Absence of an apolipoprotein E epsilon4 allele is associated with increased parietal regional cerebral blood flow asymmetry in Alzheimer disease.Arch Neurol 1998; 55: 1460–1466.
Stern Y, Brandt J, Albert M, et al. The absence of an apolipoprotein ε4 allele is associated with a more aggressive form of Alzheimer disease.Ann Neurol 1997; 41: 615–620.
Soininen H, Partanen K, Pitkanen A, et al. Decreased hippocampal volume asymmetry on MRIs in nondemented elderly subjects carrying the apolipoprotein E ε4 allele.Neurology 1995; 45: 391–392.
Tohgi H, Takahashi S, Kato E, et al. Reduced size of right hippocampus in 39- to 80-year-old normal subjects carrying the apolipoprotein E ε4 allele.Neurosci Lett 1997; 236: 21–24.
Lehtovirta M, Soininen H, Laakso MP, et al. SPECT and MRI analysis in Alzheimer’s disease: relation to the apolipoprotein E ε4 allele.J Neurol Neurosurg, Psychiatry 1996; 60: 644–649.
Arai H, Higuchi S, Muramatsu T, Iwatsubo T, Sasaki H, Trojanowski JQ. Apolipoprotein E gene in diffuse Lewy body disease with or without co-existing Alzheimer’s disease.Lancet 1994; 344: 1307.
Albin RL, Minoshima S, D’Amato CJ, Frey KA, Kuhl DA, Sima AA. Fluoro-deoxyglucose positron emission tomography in diffuse Lewy body disease.Neurology 1996; 47: 462–466.
Francis PT, Palmar AM, Snape M, Wlcock GK. The cholinergic hypothesis of Alzheimer’s disease: a review of progress.J Neurol Neurosurg Psychiatry 1999; 66: 137–147.
Rogers SL, Farlow MR, Doody RS, et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease.Neurology 1998; 50: 136–145.
Rogers SL, Doody RS, Mohs R, et al. Donepezil improves cognition and global function in Alzheimer disease.Arch Intern Med 1998; 158: 1021–1031.
Warren S, Hier DB, Pavel D. Visual form of Alzheimer’s disease and its response to anticholinesterase therapy.J Neuroimaging 1998; 8: 249–252.
Mega MS, Dinov ID, Lee L, et al. Orbital and dorsolateral frontal perfusion defect associated with behavioral response to cholinesterase inhibitor therapy in Alzheimer’s disease.J Neuropsychiatry Clin Neurosic 2000; 12: 209–218.
Staff RT, Gemmell HG, Shanks MF, Murray AD, Venneri A. Changes in the rCBF images of patients with Alzheimer’s disease receiving Donepezil therapy.Nucl Med Commun 2000; 21: 37–41.
Irie T, Fukushi K, Akimoto Y, et al. Design and evaluation of radioactive acetylcholine analogs for mapping brain acetylcholinesterase (AchE)in vivo.Nucl Med Biol 1994; 21: 801–808.
Kuhl DE, Koeppe RA, Minoshima S, et al.In vivo mapping of cerebral acetylcholinestrase activity in aging and Alzheimer’s disease.Neurology 1999; 52: 691–699.
Shinotoh H, Namba H, Fukushi K, et al. Progressive loss of cortical acetylcholinesterase activity in association with cognitive decline in Alzheimer’s disease: a positron emission tomography study.Ann Neurol 2000; 48: 194–200.
Kuhl DE, Minoshima S, Frey KA, Foster NL, Kolbourn MR, Koeppe RA. Limited donepezil inhibition of acetylcholinesterase measured by positron emission tomography in living Alzheimer cerebral cortex.Ann Neurol 2000; 48: 391–395.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsuda, H. Cerebral blood flow and metabolic abnormalities in Alzheimer’s disease. Ann Nucl Med 15, 85–92 (2001). https://doi.org/10.1007/BF02988596
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02988596