Abstract
Quenching refers to the process of rapidly cooling metal parts from the austenitizing or solution treating temperature, typically in the range of 845 to 870°C (1550 to 1600°F) for carbon and low alloy steels, for the purpose of forming martensite. Several factors determine whether a particular part can be successfully hardened, including the type, molecular weight, and thermal characteristics of the quenchant, the quenchant use conditions (such as velocity, temperature, and polymer concentration, etc.), section thickness of the part, and the transformation characteristics of the specific alloy being quenched. Successful hardening usually means achieving the required hardness, strength, or toughness while minimizing residual stress, distortion, and the possibility of cracking.
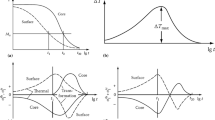
Heat removal from parts during quenching can be described in terms of the effective interface heat transfer coefficient. A quenchant must impart a sufficiently high interface heat transfer coefficient to produce a cooling rate that will minimize transformation of austenite to ferrite or pearlite and yield the desired amount of martensite.
A quench factorQ has been devised that interrelates quenching variables and transformation kinetics of steel to provide a single number indicating the extent to which a part can be hardened at various locations within the part. The quenchant and the quenchant operating conditions should be selected to provide the proper quench factor while minimizing thermal gradients that can cause distortion or cracking.
Cooling curves, interface heat transfer coefficients, quench factors, and thermal gradients produced by a variety of quenchants are presented.
Similar content being viewed by others
References
D.S. Clark and W.R. Varney,Physical Metallurgy, D. Van Nostrand Company, Inc., New York, NY, 1962, p. 170.
Atlas of Isothermal Transformation Diagrams, 3rd Ed., published by U.S. Steel, Pittsburgh, PA.
Atlas of Isothermal Transformation and Cooling Transformation Diagrams, ASM, 1979.
Atkins, M.,Atlas of Continuous Cooling Transformation Diagrams for Engineering Steels, ASM, 1980.
Austenite Transformation Kinetics of Ferrous Alloys, Vols. I and II, published by Climax Molybdenum Company, Greenwich, CT.
H.S. Carslaw and J.C. Yaeger,Conduction of Heat in Solids, Oxford University Press, Great Britain, 1959, pp. 188–213.
J.H. Hollomon and L.D. Jaffe,Ferrous Metallurgical Design, John Wiley and Sons, New York, 1947, p. 176.
E. Scheil,Arch. Eisenhuttenwes., Vol. 12, 1935, pp. 565–567.
Melvin Avrami, Kinetics of Phase Change, I,Journal of Chemical Physics, Vol. 7, December, 1939.
Melvin Avrami, Kinetics of Phase Change, II,Journal of Chemical Physics, Vol. 8, February 1940.
J.W. Cahn, The Kinetics of Grain Boundary Nucleated Reactions,Acta Metallurgica, Vol. 4, September 1956.
J.W. Cahn, Transformation Kinetics During Continuous Cooling,Acta Metallurgica, Vol. 4, November 1956.
J.H. Hollomon and L.D. Jaffe,Ferrous Metallurgical Design, John Wiley and Sons, New York, 1947, p. 39.
J.S. Kirkaldy, B.A. Thomason, and E.G. Beganis, Prediction of Multicomponent Equilibrium and Transformation for Low Alloy Steel,Hardenability Concepts with Applications to Steel, American Institute of Mining, Metallurgical and Petroleum Engineers, Inc., Warrendale, PA, 1978, p. 82.
M. Umemoto, N. Komatsubara and I. Tamura, Prediction of Hardenability Effects from Isothermal Transformation Kinetics,J. of Heat Treating, Vol. 1, No. 3, June 1980.
M. Umemoto, N. Nishioka and I. Tamura, Prediction of Hardenability from Isothermal Transformation Diagrams,J. of Heat Treating, Vol. 2, No. 2, December 1981, pp. 130–138.
J.W. Evancho and J.T. Staley, Kinetics of Precipitation in Aluminum Alloys During Continuous Cooling,Metallurgical Transactions, Vol. 5, January 1974, pp. 43–47.
Metals Handbook, Ninth Edition, Heat Treating of Aluminum Alloys, Vol. 4,Heat Treating, November 1981, pp. 675–718.
John E. Hatch, ed.,Aluminum—Properties and Physical Metallurgy, American Society for Metals, Metals Park, OH, 1984.
Morris Cohen, The Strengthening of Steel,Trans, of the Metallurgical Society of AIME, Vol. 224, August 1962, pp. 638–656.
Thelning, K.E.,Steel and Its Heat Treatment, Butter-worth & Company, London, England, 1975, p. 584.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bates, C.E. Predicting properties and minimizing residual stress in quenched steel parts. J. Heat Treating 6, 27–45 (1988). https://doi.org/10.1007/BF02833162
Issue Date:
DOI: https://doi.org/10.1007/BF02833162