Summary
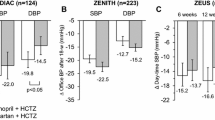
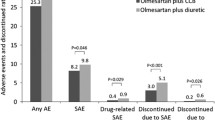
The antihypertensive activity and safety of losartan, a specific and selective antogonist of angiotensin II (subtype 1) receptors, was evaluated in 100 inpatients with mild to moderate essential hypertension. After a 2-week, single-blind, out patient placebo lead-in period, the last 2 days of which included inpatient monitoring of baseline blood pressure, the patients were assigned randomly to receive once-daily doses of either placebo; 50, 100, or 150 mg losartan; or 10 mg enalapril. Patients were treated double blind for 5 days, followed by a day for the study of drug withdrawal. Beginning with the first dose, the three doses of losartan and enalapril significantly decreased peak and trough systolic and diastolic blood pressures compared with placebo (p<-0.05). The area under the blood pressure curve was analyzed as an assessment of total blood pressure change throughout the day. On day 1, total blood pressure reduction with losartan (50–150 mg) was slightly less than with enalapril. By day 5 of double-blind treatment, the reduction in blood pressure in these groups was similar, suggesting that losartan has a slower onset of action than enalapril. No rebound hypertension was observed after study-drug discontinuation. Losartan was well tolerated in this trial, with an adverse event profile similar to placebo and enalapril.
Similar content being viewed by others
References
GarrisonJC, PeachMJ. Renin and angiotensin. In GilmanAG, RallTW, NiesAS, TaylorP, eds.Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed. New York: McGraw-Hill, 1990:749–763.
EhiersMRW, RiordanJF. Angiotensin-converting enzyme: Biochemistry and molecular biology. In LaraghJH, BrennerBM, eds.Hypertension, Pathophysiology, Diagnosis and Management. New York: Raven Press, 1990:1217–1231.
KarlbergBE. Cough and inhibition of the renin-angiotensin system.J Hypertens 1993;11(Suppl 3):S49-S52.
LacourciereY, LeFebvreJ, NakhleG, FaisonEP, SnavelyDB, NelsonEB. Association between cough and angiotensin converting enzyme inhibitors vs. angiotensin II antagonists: The design of a prospective, controlled study.J Hypertens 1994;12(Suppl 2):S49-S53.
SieglPKS. Discovery of losartan, the first specific nonpeptide angiotensin II receptor antagonist.J Hypertens 1993;11(Suppl 3):S19-S22.
SmithRD, ChiuAT, WongPC, HerblinWF, TimmermansPBMWM. Pharmacology of nonpeptide angiotensin II receptor antagonists.Annu Rev Pharmacol Toxicol 1992;32: 135–165.
TimmermansPBMWM, BenfieldP, ChiuAT, HerblinWF, WongPC, SmithRD. Angiotensin II receptors and functional correlates.Am J Hypertens 1992;5:221S-235S.
WongPC, PriceWJ, ChiuAT, et al. In vivo pharmacology of DuP 753.Am J Hypertens 1991;44:228S-298S.
CockcroftJR, SciberrasDG, GoldbergMR, RitterJM. Comparison of angiotensin-converting enzyme inhibition with angiotensin II receptor antagonism in the human forearm.J Cardiovasc Pharmacol 1993;22:579–584.
ChristenY, WaeberB, NussbergerJ, et al. Oral administration of DuP 753, a specific angiotensin II receptor antagonist, to normal male volunteers. Inhibition of pressor response to exogenous angiotensin I and II.Circulation 1991; 83:1333–1342.
ChristenY, WaeberB, NussbergerJ, LeeRJ, TimmermansPBMWM, BrunnerHR. Dose-response relationship following oral administration of DuP 753 to normal humans.Am J Hypertens 1991;4:350S-353S.
MunafoA, ChristenY, NussbergerJ, et al. Drug concentration response relationships in normal volunteers after oral administration of losartan, an angiotensin II receptor antagonist.Clin Pharmacol 1992;51:513–521.
BrunnerDB, DespondsG, BiollazJ, et al. Effect of a new angiotensin converting enzyme inhibitor MK 421 and its lysine analogue on the components of the renin system in healthy subjects.Br J Clin Pharmacol 1981;11:461–467.
GavrasH, BiollazJ, WaeberB, BrunnerHR, GavrasI, DaviesRO. Antihypertensive effect of the new oral angiotensin converting enzyme inhibitor MK-421.Lancet 1981;2: 543–547.
GradmanAH, ArcuriK, GoldbergAI, IkedaLS, NelsonEB, SnavelyDB, SweetCS. A randomized, placebo-controlled, double-blind parallel study of various doses of losartan potassium compared to enalapril maleate in patients with essential hypertension.Hypertension 1995;25: 1339–1344.
KeimHJ, DrayerJI, CaseDB, et al. A role for renin in rebound hypertension and encephalopathy after infusion of saralasin acetate (Sar1Ala8-angiotensin II).N Engl J Med 1976;295:1175–1177.
GoldbergAI, DunlayMC, SweetCS. The safety and tolerability of losartan potassium, and angiotensin II receptor antagonist in comparison to hydrochlorothiazide, atenolol, felodipine ER and ACE inhibitors for the treatment of systemic hypertension.Am J Cardiol 1995;75:793–795.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Byyny, R.L., Merrill, D.D., Bradstreet, T.E. et al. An inpatient trial of the safety and efficacy of losartan compared with placebo and enalapril in patients with essential hypertension. Cardiovasc Drug Ther 10, 313–319 (1996). https://doi.org/10.1007/BF02627955
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02627955