Abstract
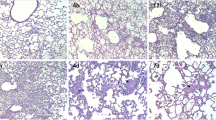
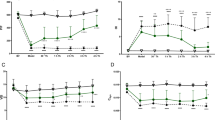
The administration of a high-dose of a serine protease inhibitor is recommended in patients complicated by multiple organ failure (MOF), including adult respiratory distress syndrome (ARDS), induced by acute pancreatitis. The accumulation of polymorphonuclear leukocytes (PMN) in affected organs is considered to be one of the causative factors of MOF. Adhesion to endothelial cells (EC), via adhesion molecules, and the transendothelial migration of PMN is closely associated with the accumulation of PMN. We examined the effects of two serine protease inhibitors, ulinastatin (UT) and gabexate mesilate (GM), on EC-PMN adhesion and transendothelial migration in human umbilical vein EC and51Cr-labeled PMN in vitro. EC-PMN adhesion, and the expression of intercellular adhesion molecule-1 (ICAM-1) and endothelial cell adhesion molecule-1 (ELAM-1) on EC induced by IL-1β and TNFα, were reduced by the pretreatment of EC with these inhibitors. The transendothelial migration of PMN stimulated by IL-8 was also inhibited by pretreating PMN with UT or GM. We also examined whether these inhibitors reduced PMN accumulation in the lung in rats with acute pancreatitis induced by a closed duodenal loop. The myeloperoxidase activity in and histological findings of the lung suggested that UT and GM reduced PMN accumulation. In conclusion, serine protease inhibitors may inhibit PMN accumulation in ARDS due to acute pancreatitis.
Similar content being viewed by others
References
Renner IG, Savage WT, Pantoja JL, et al. Death due to acute pancreatitis: A retrospective analysis of 405 autopsy case. Dig Dis Sci 1985;30:1005–1018.
Lankish PG, Rahlf G, Koop H. Pulmonary complications in fatal acute hemorrhagic pancreatitis. Dig Dis Sci 1983;28: 111–116.
Ratliff NB, Wilson JW, Mikat E, et al. The lung in hemorrhagic shock. IV. The role of the polymorphonuclear leukocyte. Am J Pathol 1971;65:325–331.
Guise KS, Oldman KT, Johnson KJ, et al. Pancreatitis-induced acute lung injury: An ARDS model. Ann Surg 1988;208:71–77.
Willemer S, Fedderson CO, Karges W, et al. Lung injury in acute pancreatitis in rat. Int J Pancreatol 1991;8:305–320.
Donnelly SC, Hastlett C. Cellular mechanism of acute lung injury; implications for future treatment in the adult respiratory distress syndrome. Thorax 1992;47:260–263.
Gamble JR, Harlan JM, Klebanoff SJ, et al. Stimulation of adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci 1985;82:8667–8671.
Bevilacqua MP, Pober JS, Wheeler ME, et al. Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest 1985;76:2003–2011.
Goldbum SE, Choen DA, Gillespie MN, et al. Interleukin-1-induced granulocytopenia and pulmonary leukostasis in rabbits. J Appl Physiol 1987;162:122–128.
Barton RW, Rothlein R, Ksiazek J, et al. The effect of antiintercellular adhesion molecule-1 on phorbol ester-induced rabbit lung inflammation. J Immunol 1989;143:1278–1282.
Mulligan MS, Varani J, Dame MK, et al. Role of endothelial-leukocyte adhesion molecule 1 (ELAM-1) in neutrophilmediated lung injury in rats. J Clin Invest 1991;88:1396–1406.
Wautier JL, Setiadi H, Vilette D, et al. Leukocyte adhesion to endothelial cells. Biorheology 1990;27:425–432.
Charles AD, Jeffrey AG, Sheldon MW. Anticytokine strategies in the treatment of the systemic inflammatory response syndrome. JAMA 1993;269:1829–1835.
Wall RT, Harker LA, Quadracci LJ, et al. Factors influencing endothelial cell proliferation in vitro. J Cell Physiol 1978; 96:203–213.
Thornton SC, Mueller SN, Levine EM. Human endothelial cells: Use of heparin in cloning and long-term serial cultivation. Science 1983;222:623–625.
Maciag T, Cerundolo J, Ilsley S et al. An endothelial cell growth factor from bovine hypothalamus: Identification and partial characterization. Proc Natl Acad Sci USA 1979;76: 5674–5678.
Harlan JM, Killen PD, Senecal FM, et al. The role of neutrophil membrane glycoprotein GP-150 in neutrophil adherence to endothelium in vitro. Blood 1985;66:167–178.
Gallin JI, Clark RA, Kimball HR. Granulocyte chemotaxis: An improved in vitro assay employing51Cr-labeled granulocytes. J Immunol 1973;110:233–240.
Liu DY, Kaymakcalan Z. In vitro adhesion assay. In: Harlan JM (ed) Adhesion—its role in inflammatory disease. New York: WH Freeman, 1992:189–193.
Leeuwenberg JFM, Von Asmuth EJU, Jeunhomme TMAA, Buurman WA. IFN-γ regulates the expression of the adhesion molecule ELAM-1 and IL-6 production by human endothelial cells in vitro. J Immunol 1990;145:2110–2114.
Yamada Y, Yokota M, Furumichi T, et al. Protective effects of calcium channel blockers on hydrogen peroxide-induced increases in endothelial permeability. Cardiovascular Res 1990; 114:993–997.
Hahn U, Stallmach A, Hahn EG, et al. Basement membrane components are potent promoters of rat intestinal epithelial cell differentiation in vitro. Gastroenterology 1990;98:322–335.
Nevelainen TJ, Seppa A. Acute pancreatitis caused by closed duodenal loop in the rat. Scand J Gastroenterol 1975;10: 521–527.
Ceska M, Birath K, Brown B. A new and rapid method for the clinical determination of alpha-amylase activities in human serum and urine. Clin Chem Acta 1969;26:437–444.
Goldbum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol 1985;59:1978–1985.
Pober JS, Gimbrone MA, Lapierre LA, et al. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol 1986;137:1893–1896.
Ishii Y, Siu KL, Malik AB. Neutrophil adhesion to TNFα-activated endothelial cells potentiates leukotriene B4 production. J Cell Biol 1992;153:187–195.
Chakraborti S, Michael JR. Role of serine esterase in A23187-mediated activation of phospholipase A2 in pulmonary endothelium. Am J Physiol 1993;264:L538-L542.
Peveri P, Walz A, Dewald B, et al. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med 1988;167:1547–1559.
Fujishima S, Sasaki J, Shinozawa Y, et al. Interleukin-8 in ARDS. Lancet 1993;342:237–238.
Aswanikumar S, Schiffmann E, Corcoran BA, et al. Role of a peptidase in phagocyte chemotaxis. Proc Natl Acad Sci USA 1976;73:2439–2442.
Kitagawa S, Takaku F, Sakamoto S. Evidence that proteases are involved in superoxide production by human PMN and monocytes. J Clin Invest 1980;65:74–81.
Tani S, Otsuki M, Itor H, et al. The protective effect of the trypsin inhibitor urinastatin on cerulein-induced acute pancreatitis in rats. Pancreas 1988;3:471–476.
Weiss SJ, Curnutte JT, Regiani S. Neutrophil-mediated solubization of the subendothelial matrix: Oxidative and non-oxidative mechanisms of proteolysis used by normal and chronic granulomatous disease phagocytes. J Immunol 1986;136: 636–641.
Stockley RA, Shaw J, Afford SC, et al. Effect of alpha-1-protease inhibitor on neutrophil chemotaxis. Am J Respir Cell Mol Biol 1990;2:163–170.
Banda MJ, Rice AG, Griffin GL, et al. α1-Proteinase inhibitor is a neutrophil chemoattractant after proteolytic inactivation by macrophage elastase. J Biol Chem 1988;263: 4481–4484.
Horiuchi K, Kanayama N. Human urinary trypsin inhibitor exposed to oxidants produced by myeroperoxidase-H2O2-chloride inhibits human neutrophil elastase. Jpn J Pharmacol Ther 1988;16:2007–2012.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Okumura, Y., Inoue, H., Fujiyama, Y. et al. Effects of serine protease inhibitors on accumulation of polymorphonuclear leukocytes in the lung induced by acute pancreatitis in rats. J Gastroenterol 30, 379–386 (1995). https://doi.org/10.1007/BF02347515
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02347515