Abstract
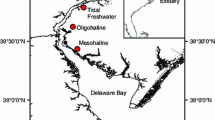
The seasonal flux of methane to the atmosphere was measured at three salt marsh sites along a tidal creek. Average soil salinities at the sites ranged from 5 to 17 ppt and fluxes ranged from below detection limits (less than 0.3 mgCH4 m-2 d-1) to 259 mgCH4 m-2 d-1. Annual flux to the atmosphere was 5.6 gCH4 m-2 from the most saline site, 22.4 gCH4 m-2 from the intermediate site, and 18.2 gCH4 m-2 from the freshest of the three sites. Regression of the amount of methane in the soil with flux indicates that changes in this soil methane can account for 64% of the observed variation in flux. Data on pore water distributions of sulfate suggests that the activity of sulfate reducing bacteria is a primary control on methane flux in these transitional environments. Results indicate that relatively high emissions of methane from salt marshes can occur at soil salinities up to approximately 13 ppt. When these data are combined with other tidal marsh studies, annual CH4 flux to the atmosphere shows a strong negative correlation with the long term average soil salinity over a range from essentially fresh water to 26 ppt.
Similar content being viewed by others
References
Abram, J.W. & D.B. Nedwell (1978) Inhibition of methanogenesis by sulfate-reducing bacteria competing for transferred hydrogen. Archives of Microbiology 117: 89–92
Alperin, M.J. & W.S. Reeburgh (1985) Inhibition experiments on anaerobic methane oxidation. Applied and Environmental Microbiology 50: 940–945
Atkinson, L.P. & J.R. Hall (1976) Methane distribution and production in the Georgia salt marsh. Estuarine and Coastal Marine Science 4: 677–686
Balderson, W.L. & W.J. Payne (1976) Inhibition of methanogenesis in salt marsh sediments and whole-cell suspensions of methanogenic bacteria by nitrogen oxides. Applied and Environmental Microbiology 32: 264–269
Bartlett, K.B., R.C. Harriss & D.I. Sebacher (1985) Methane flux from coastal salt marshes. Journal of Geophysical Research 90: 5710–5720
Berner, R.A. (1980) Early Diagenesis. Princeton University Press, Princeton, New Jersey, USA
Cicerone, R.J. & J.D. Shetter (1981) Sources of atmospheric methane: Measurements in rice paddies and a discussion. Journal of Geophysical Research 86: 7203–7209
Craig, H. & C.C. Chou (1982) Methane: The record in polar ice cores. Geophysical Research Letters 9: 1221–1224
Crill, P.M. & C.S. Martens (1983) Spatial and temporal fluctuations of methane production in anoxic coastal marine sediments. Limnology and Oceanography 28: 1117–1130
Dacey, J.W.H. (1981) Pressurized ventilation in the yellow waterlily. Ecology 62: 1137–1147
Dacey, J.W.H. & M.J. Klug (1979) Methane efflux from lake sediments through water lilies. Science 203: 1253–1254
Denmead, O.T. (1979) Chamber systems for measuring nitrous oxide emission from soils in the field. Soil Science Society America Journal 43: 89–95
DeLaune, R.D., C.J. Smith & W.H. Patrick (1983) Methane release from Gulf coast wetlands. Tellus 35B: 8–15
Devol, A.H. (1983) Methane oxidation rates in the anaerobic sediments of Saanich Inlet. Limnology and Oceanography 28: 738–742
Ehhalt, D.H. & U. Schmidt (1978) Sources and sinks of atmospheric methane. Pure and Applied Geophysics 116: 452–464
Gallagher, J.L. & F.G. Plumley (1979) Under-ground biomass profiles and productivity in Atlantic coastal marshes. American Journal of Botany 66: 156–161
Good, R.E., N.F. Good & B.R. Frasco (1982) A review of primary production and decomposition dynamics of the below-ground marsh component. In: V.S. Kennedy (Ed) Estuarine Comparisons (pp. 139–157) Academic Press, New York, New York, USA
Graedel, T.E. & J.E. McRae (1980) On the possible increase of the atmospheric methane and carbon monoxide concentrations during the last decade. Geophysical Research Letters 7: 977–979
Harriss, R.C. & D.I. Sebacher (1980) Reassessing the importance of wetlands as a global source of atmospheric methane. Eos Transactions, AGU 61: 239
Harriss, R.C. & D.I. Sebacher (1981) Methane flux in forested freshwater swamps of the southeastern United States. Geophysical Research Letters 8: 1002–1004
Harriss, R.C., D.I. Sebacher & F.P. Day (1982) Methane flux in the Great Dismal Swamp. Nature 297: 673–674
Hesslein, R.H. (1976) An in situ sampler for close interval pore water studies. Limnology and Oceanography 21: 912–914
Hines, M.E. & J.D. Buck (1982) Distribution of methanogenesis and sulfate-reducing bacteria in near-shore marine sediments. Applied and Environmental Microbiology 43: 447–453
Howarth, R.W. & J.M. Teal (1979) Sulfate reduction in a New England salt marsh. Limnology and Oceanography 24: 999–1013
Howes, B.L., J.W.H. Dacey & J.M. Teal (1985) Annual carbon mineralization and belowground production ofSpartina alterniflora in a New England salt marsh. Ecology 66: 595–605
Iversen, N. & B.B. Jorgensen (1985) Anaerobic methane oxidation rates at the sulfate-methane transition in marine sediments from Kattegat and Skagerrak (Denmark). Limnology and Oceanography 30: 944–955
Jones, J.G., B.M. Simon & S. Gardener (1982) Factors affecting methanogenesis and associated anaerobic processes in the sediments of a stratified eutrophic lake. Journal of General Microbiology 128: 1–12
Kelly, C.A. & D.P. Chynoweth (1981) The contributions of temperature and of the input of organic matter in controlling rates of sediment methanogenesis. Limnology and Oceanography 26: 891–897
Khalil, M.A.K. & R.A. Rasmussen (1986) Interannual variability of atmospheric methane: Possible effects of the El Nino—Southern Oscillation. Science 232: 56–58
King, G.M. & W.J. Wiebe (1978) Methane release from soils of a Georgia salt marsh. Geochimica et Cosmochimica Acta 42: 343–348
Lacis, A., J. Hansen, P. Lee, T. Mitchell & S. Lebedeff (1981) Greenhouse effect of trace gases, 1970–1980. Geophysical Research Letters 8: 1035–1038
Liss, P.S. & P.G. Slater (1974) Flux of gases across the air-sea interface. Nature 247: 181–184
Logan, J.A., M.J. Prather, S.C. Wofsy & M. McElroy (1981) Tropospheric chemistry: A global perspective. Journal of Geophysical Research 86: 7210–7254
Mah, R.A., D.M. Ward, L. Baresi & T.L. Glass (1977) Biogenesis of methane. Annual Review of Microbiology 31: 309–341
Martens, C.S. & R.A. Berner (1977) Interstitial water chemistry of anoxic Long Island Sound sediments. I. Dissolved gases. Limnology and Oceanography 22: 10–25
Martens, C.S. & M.B. Goldhaber (1978) Early diagenesis in transitional sedimentary environments of the White Oak River Estuary, North Carolina. Limnology and Oceanography 23: 428–441
Martens, C.S. & J.V. Klump (1980) Biogeochemical cycling in an organic-rich coastal basin. I. Methane sediment-water exchange processes. Geochimica et Cosmochimica Acta 44: 471–490
Matthias, A.D., A.M. Blackmer & J.M. Bremner (1980) A simple chamber technique for field measurement of emissions of nitrous oxide from soils. Journal of Environmental Quality 9: 251–256
McAuliffe, C. (1971) Gas chromatographic determination of solutes by multiple phase equilibrium. Chemical Technology 1: 46–51
Nixon, S.W. (1980) Between coastal marshes and coastal waters—A review of twenty years of speculation and research on the role of salt marshes in estuarine productivity and water chemistry. In: P. Hamilton & K.B. MacDonald (Eds) Estuarine and Wetland Processes (pp. 437–525) Plenum Press, New York, New York, USA
Oremland, R.S. & B.F. Taylor (1978) Sulfate reduction and methanogenesis in marine sediments. Geochimica et Cosmochimica Acta 42: 209–214
Oremland, R.S. & S. Polcin (1982) Methanogenesis and sulfate reduction: Competitive and noncompetitive substrates in estuarine sediments. Applied and Environmental Microbiology 44: 1270–1276
Rasmussen, R.A. & M.A.K. Khalil (1981) Atmospheric methane (CH4): Trends and seasonal cycles. Journal of Geophysical Research 86: 9826–9832
Reeburgh, W.S. & D.T. Heggie (1977) Microbial methane consumption reactions and their effect on methane distributions in freshwater and marine environments. Limnology and Oceanography 22: 1–9
Rudd, J.W., R.D. Hamilton & N.E. Campbell (1974) Measurement of microbial oxidation of methane in lake water. Limnology and Oceanography 19: 519–524
Rudd, J.W.M. & C.D. Taylor (1980) Methane cycling in aquatic environments. Advances in Aquatic Microbiology 2: 77–150
Schubauer, J.P. & C.S. Hopkinson (1984) Above- and below-ground emergent macrophyte production and turnover in a coastal marsh ecosystem, Georgia. Limnology and Oceanography 29: 1052–1065
Sebacher, D.I. (1985) Nondispersive infrared absorption monitors for trace gases. In: J. Wormhoudt (Ed) Infrared Methods for Gaseous Measurements: Theory and Practice (pp. 248–274) Marcel Dekker, Inc., New York, New York, USA
Sebacher, D.I. & R.C. Harriss (1982) A system for measuring methane fluxes from inland and coastal wetland environments. Journal of Environmental Quality 11: 34–37
Sebacher, D.I., R.C. Harriss & K.B. Bartlett (1985) Methane emissions to the atmosphere through aquatic plants. Journal of Environmental Quality 14: 40–46
Seiler, W., A. Holzapfel-Pschorn, R. Conrad & D. Scharffe (1984) Methane emission from rice paddies. Journal of Atmospheric Chemistry 1: 241–268
Silberhorn, G.M. (1981) York County and Town of Poquoson Tidal Marsh Inventory, 2nd edn. Virginia Institute of Marine Science Special Report # 53 in Applied Marine Science and Ocean Engineering
Smalley, A.E. (1959) The role of two invertebrate populations,Littorina irrorata andOrchelium fzducinum, in the energy flow of a salt marsh ecosystem. Ph.D. dissertation, University of Georgia, Athens, Georgia
Stauffer, B., G. Fischer, A. Neftel & H. Oeschger (1985) Increase of atmospheric methane recorded in Antarctic ice core. Science. 229: 1386–1388
Stevens, C.M. & F.E. Rust (1982) The carbon isotope composition of atmospheric methane. Journal of Geophysical Research 87: 4879–4882
winfrey, M.R. & J.G. Zeikus (1977) Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Applied and Environmental Microbiology 33: 275–281
Zehnder, A.J.B. (1978) Ecology of methane formation. In: R. Mitchell (Ed) Pollution Microbiology, Vol. 2 (pp. 349–376) John Wiley and Sons, New York, New York, USA
Zeikus, J.G. & M.R. Winfrey (1976) Temperature limitation of methanogenesis in aquatic sediments. Applied and Environmental Microbiology 31: 99–107
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bartlett, K.B., Bartlett, D.S., Harriss, R.C. et al. Methane emissions along a salt marsh salinity gradient. Biogeochemistry 4, 183–202 (1987). https://doi.org/10.1007/BF02187365
Issue Date:
DOI: https://doi.org/10.1007/BF02187365