Summary
-
1.
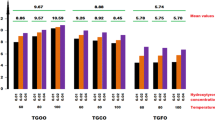
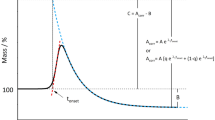
Accumulation of peroxides during oxidation of a fat takes place in two stages. The first stage is a relatively slow reaction with weak auto-acceleration and an activation energy of 20–25 kcal. The second stage goes with high velocity until the concentration of peroxide reaches a maximum, and its activation energy is 14.5 kcal.
-
2.
Addition of tart -butylhydroxyanisole considerably lengthens the first stage of oxidation without influencing its activation energy.
-
3.
Introduction of butylhydroxyanisole at the second stage sharply inhibits the oxidation. After a short period, however, the reaction is renewed at high velocity evidently due to destruction of the inhibitor.
-
4.
The occurrence of two stages of oxidation is evidently associated with the presence of a natural antioxidant in the fat, the destruction of this antioxidant leading to commencement of rapid oxidation.
Similar content being viewed by others
Literature cited
N. Emanuel, D. Knorre, Yu. Lyaskovskaya and V. Piulskaya, Meat Industry, No. 5, 44 (1955).
N. Emanuel, D; Knorre, Yu. Lyaskovskaya and V. Piulskaya, Meat Industry, No. 6, 47 (1955).
A. A. Zinovyev, Chemistry of Fats (Food Industry Press, Moscow, 1952).
J. L. Bolland and G. Gee, Trans. Faraday Soc. 42, 236 (1946).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Knorre, D.G., Lyaskovskaya, Y.N. & Emanuel, N.M. The kinetics of oxidation of fats. Russ Chem Bull 6, 693–698 (1957). https://doi.org/10.1007/BF01167219
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01167219